
Applied Clinical Trials
Clinical professionals can recreate CTMS by applying modern technology in a new way.

Applied Clinical Trials
Clinical professionals can recreate CTMS by applying modern technology in a new way.

Applied Clinical Trials
INFORMATION TECHNOLOGY : Revolution in Randomization Clinical Database: Proper Coding and Design Also in this issue : PDUFA Renewal, EFGCP Meeting on Informed Consent, Drug Development Cycle Time, Choosing an eClinical Solution

Applied Clinical Trials
Congress, industry map out goals and concerns for revising FDA policies linked to user fee legislation.

Applied Clinical Trials
The FDA issued guidances in February that outlines its recommendations for developing and approving biosimilar therapies.


Applied Clinical Trials
The clinical trials industry can benefit from site-driven metrics for process improvement and site scoring.

Applied Clinical Trials
Obsession with shorter cycle times has been mistakenly drilled into all aspects of development.

Applied Clinical Trials
One of the biggest obstacles to Alzheimer's research is recruitment and retention.

Applied Clinical Trials
European Forum for Good Clinical Practice meeting on informed consent draws a huge crowd.

Applied Clinical Trials
The European Patients' Academy on Therapeutic Innovation faces its first major test during the 2012 Drug Information Association EuroMeeting.

Applied Clinical Trials
Joseph Kim discusses a recent patient panel he moderated.
Applied Clinical Trials
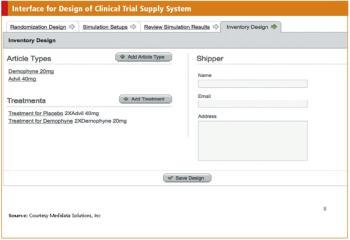
Systems that support automated processes have begun to transform the conduct of clinical trials.

Applied Clinical Trials
Positive patient experiences can help drive late phase study success.

Applied Clinical Trials
Choosing eClinical solutions can appear to be a tricky situation.



Applied Clinical Trials
Proper questionnaire coding and design can help set the stage for a successful trial.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.