
Applied Clinical Trials
Outlining the latest government, industry, and public health efforts to promote increased adoption of common standards in data collection and sharing.

Applied Clinical Trials
Outlining the latest government, industry, and public health efforts to promote increased adoption of common standards in data collection and sharing.

Applied Clinical Trials
FDA released four new draft guidances that seek to broaden criteria for identifying and enrolling patients in clinical trials in an effort to reduce time and cost of clinical research for biopharmaceuticals.

Applied Clinical Trials
A look at the scope of the agency’s draft framework for evaluating the use of RWE to support new drug approvals and the implications for sponsors.

Applied Clinical Trials
A statement from Europe’s drug industry and Europe’s medical societies calls for a “bold vision” to ensure proper funding and coordination for translational research.

Applied Clinical Trials
CROs should be more mindful of their smaller emerging biopharma clients and pay more attention to handling their needs.

Applied Clinical Trials
The “citizen data scientist” is the person with no official data scientist training who uses the latest tools and technologies to handle data wrangling duties, analyze data, and create reports and models.
Applied Clinical Trials
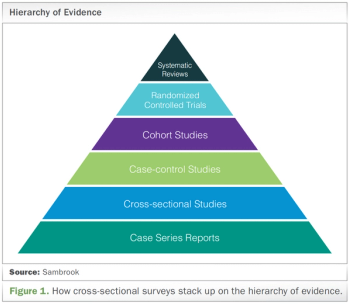
With outcomes from “real life” a critical compliment to clinical trial data, the importance of involving an epidemiologist at study inception is explored.

Applied Clinical Trials
A leading industry executive in the regenerative medicine space discusses recent advances in stem cell therapy development.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials April 2019 issue in an interactive PDF format.