Applied Clinical Trials
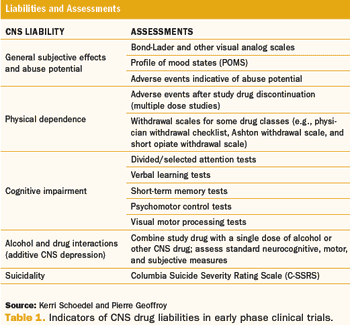
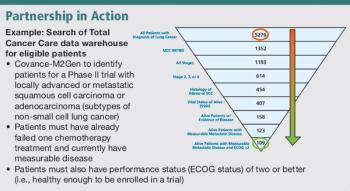
Subject Recruitment: The Ideas Initiative Trial Design: Early Phase CNS Drug Liabilities; Improving Enrollment Forecasts Also in this issue: Update on Europe's Clinical Trial Rules Debate Public Perception of Clinical Research Clinical Trial Barriers