
Applied Clinical Trials
Comparative drug analysis aims to address costs and value as candidates eye curbs on drug spending.

Applied Clinical Trials
Comparative drug analysis aims to address costs and value as candidates eye curbs on drug spending.
Applied Clinical Trials
Insurty gets ready to meet FDA’s requirement that regulatory submissions be in eCTD format.
Applied Clinical Trials
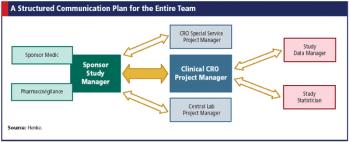
Just like pharma, biotech companies need clinical research organizations too, but are their needs different?

Applied Clinical Trials
A new initiative aims to identify new methods to make the clinical trials process more efficient and effective.

Applied Clinical Trials
Acceptance of data ambiguity by the industry is the key to adaptive design adoption and use.

Applied Clinical Trials
A new project focuses on developing Africa's legal and regulatory framework for health research.
Applied Clinical Trials
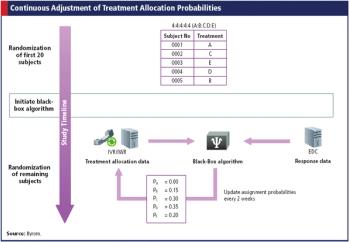
With help from technology, adaptive trials can enhance dose selection and reduce time between phases.

Applied Clinical Trials
Chris Bode, PhD, vice president of corporate development for Absorption Systems, explains the impact of in vitro models relative to the Critical Path Initiative.

Applied Clinical Trials
In the European Union, regulators are agonizing over more than just clinical trials.

Applied Clinical Trials
DIA expands into India with a new office and advisory council.

Applied Clinical Trials
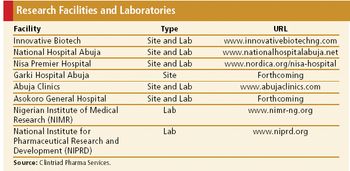
Low costs, a rich patient base, and strong talent pool characterize Africa's most populated country.

Applied Clinical Trials
Why this age-old industry staple is missing the mark for many and what can be done to remedy the situation.

Applied Clinical Trials
Survey uncovers current market and future directions for the European clinical trials industry.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
How one workload study measured tasks, time, and resources necessary to run a cancer clinical trial today.
Applied Clinical Trials
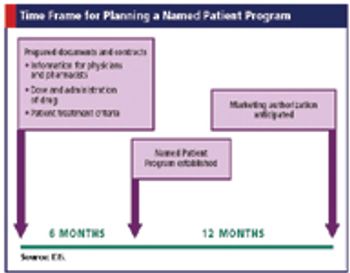
Integrating clinical trials and named patient programs to provide global access to drugs before approval.
Applied Clinical Trials
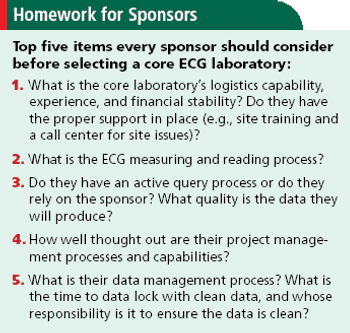
A call to sponsors to rethink the role of ECGs in drug development and the use of central core labs.
Applied Clinical Trials
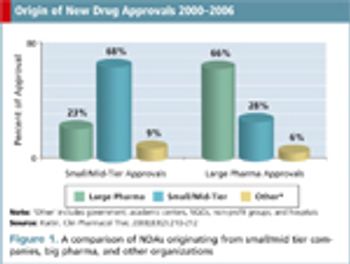
CROs must adjust their practices to cater to small/mid-tier pharma instead of simply servicing big pharma.

Applied Clinical Trials
A look at its early beginnings, where it is today, and what the future could hold.

Applied Clinical Trials
Pilot test to rebrand clinical research shows promise as a way to build public trust and promote interest.