
Applied Clinical Trials
Cost inefficiencies in drug development are a major focus of discussion, especially with the many financial impacts affecting the pharmaceutical sponsors.

Applied Clinical Trials
Cost inefficiencies in drug development are a major focus of discussion, especially with the many financial impacts affecting the pharmaceutical sponsors.


Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
The percentage of source document verification coverage has been decreasing slightly over the past four years. Therefore, the amount of on-site time required by site monitors should also start decreasing.

Applied Clinical Trials
Proposed rule changes on public information in Europe never make it to fruition.
Applied Clinical Trials
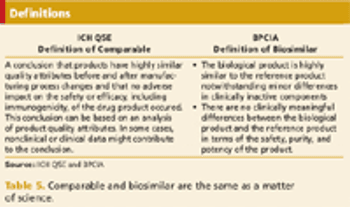
Biosimilars are a steadily growing new field of biopharmaceutical development and clinical research.
Applied Clinical Trials
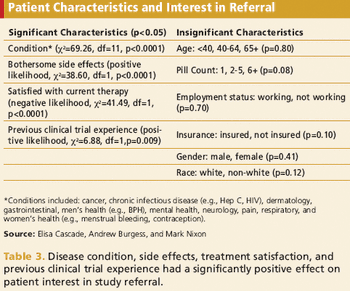
Analysis suggests age, condition, and treatment satisfaction have most significant effect on participation.

Applied Clinical Trials
In August last year, the FDA came out with its Guidance for Industry Oversight of Clinical Investigations-a Risk-Based Approach to Monitoring. Sponsors and CROs alike are still examining the document and discussing how best to move forward.

Applied Clinical Trials
Criticisms of the pharmaceutical industry's real motives for undertaking post-marketing studies have been made by a special report in the British Medical Journal.

Applied Clinical Trials
The main result of the Supreme Court decision upholding the Affordable Care Act (ACA) is that it ends all the rampant speculation and uncertainty about the future shape of the US healthcare system.

Applied Clinical Trials
Despite their growing role, little benchmark management data, until now, existed on global study monitors.

Applied Clinical Trials
Prior to initiating a trial with ED sites, three factors must be considered in order to achieve success.

Applied Clinical Trials
It's hard enough to conduct clinical trials for experimental medicines, but it can be even more difficult when patients already have access to the medicine outside the research setting.

Applied Clinical Trials
Regulatory: Developing Biosimilars for a Global Market Subject Recruitment: Patient Interest in Clinical Trial Referral CRO/Sponsor: Functional Service Provider Model Also in this issue: Proposed Rule Changes in Europe Global Study Monitors Benchmarks Emergency Department Trials