
Applied Clinical Trials
Pressure to shorten study start-up timelines puts clinical supply management in the crosshairs.

Applied Clinical Trials
Pressure to shorten study start-up timelines puts clinical supply management in the crosshairs.
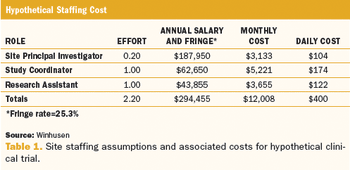
Applied Clinical Trials
Method designed to reduce the time gap between protocol approval and recruitment is examined.

Applied Clinical Trials
New phase of innovation initiative emphasizes focus on research networks, adaptive trials, and personalized medicine, among other key areas.

Applied Clinical Trials
eGSP-driven approach focuses on overcoming operational challenges through data integration.

Applied Clinical Trials
Consumer groups, research organizations, and Congress are pressing for more inclusive clinical research.
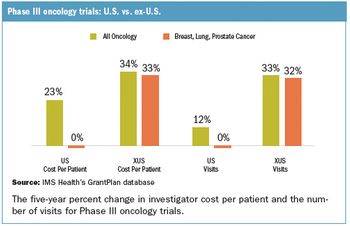
Applied Clinical Trials
IMS Health

Applied Clinical Trials
Longer-term strategic issues are making already high-investment, high-risk decisions to place studies overseas even more complex.

Applied Clinical Trials
Unique feedback from patient survey could help inform future clinical supply design and implementation.
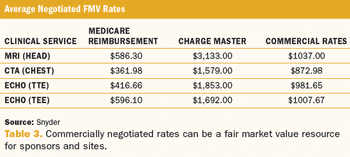
Applied Clinical Trials
Breaking through the confusion around fair market value when applying the concept to study budgets.

Applied Clinical Trials
The European Forum for Good Clinical Practice says proposed restrictive access to clinical data would impede further progress in health research.

Applied Clinical Trials
CRO/Sponsor: Clinical Supply Survey, Strategies Sites: Fair Market Value Debate Clinical Technology: Optimizing Supply Chain Data Trial Design: Multi-site Pre-implementation Also in this issue: European Innovation Initiative, Part II Medicine Kits: The Patient Perspective Global Trials Need Strategic Touch