
Applied Clinical Trials
The agency is encouraging drug companies to adopt innovative research methods and development tools-and showing more flexibility in approving therapies that have taken non-conventional paths.

Applied Clinical Trials
The agency is encouraging drug companies to adopt innovative research methods and development tools-and showing more flexibility in approving therapies that have taken non-conventional paths.

Applied Clinical Trials
Pressure is growing on clinical trial organizers to involve more pregnant women in drug research.

Applied Clinical Trials
The European Health Data Network (EHDN) initiative kicks off-a challenging and resource-intensive effort designed to harmonize data analysis and conversion amid the push toward outcomes-focused healthcare in Europe.

Applied Clinical Trials
Q&A explores the importance of new vehicles to identify and recruit physicians seeking non-interventional research opportunities.
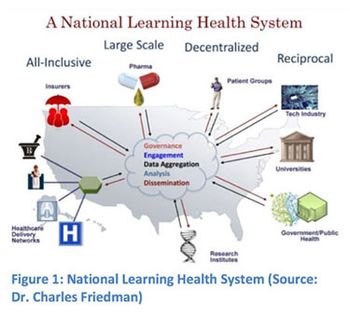
Applied Clinical Trials
Outlining the ways FDA is adopting electronic practices and initiating efforts to simplify the submission process for new drug and generic drug applications.

Applied Clinical Trials
Q&A discusses how the bridging of scientific merit with the needs of patients can lead to successful clinical development.

Applied Clinical Trials
Traditional and new players are seeing growth and opportunity as the integration of clinical research and healthcare intensifies.

Applied Clinical Trials
As industry headlines buzz with drug pricing uncertainty and M&A speculation, sponsors can boost productivity on the R&D front through more conscious management of outsourcing spend.
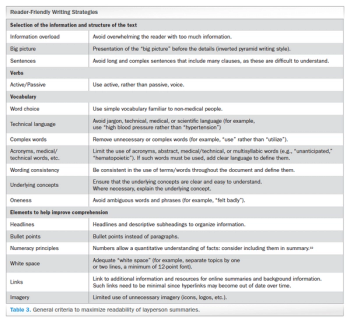
Applied Clinical Trials
Analyzing the debate around writing and constructing layperson summaries of clinical trial results-and proposing a “reader-centered” approach.
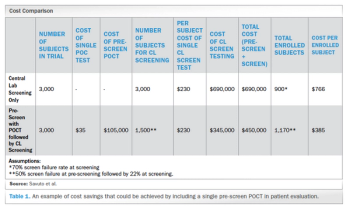
Applied Clinical Trials
Retrospective analysis of clinical trial enrollment data evaluates the effectiveness of point-of-care testing in reducing later-screen failures.

Applied Clinical Trials
New survey data, coupled with recent research, spotlight the current views and concerns around multi-regional studies.

Applied Clinical Trials
Amid increased consolidation in the CRO space, more biopharma alliances, and the emergence of new clinical technology platforms, finding the formula to turn all these good intentions into tangible improvements in drug development practice remain elusive.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials July/August 2017 issue in an interactive PDF format.