
Applied Clinical Trials
Despite orphan-drug R&D and approval being all the rage, issues such as patient access could leave these treatments out in the rain.

Applied Clinical Trials
Despite orphan-drug R&D and approval being all the rage, issues such as patient access could leave these treatments out in the rain.

Applied Clinical Trials
The discussions will center on ways clinical research can harness gene therapy and new and emerging diagnostic tools.

Applied Clinical Trials
Exploring three pivotal technology areas that could reshape global studies over the next 10 years.

Applied Clinical Trials
While approved in 2014, Europe's new regulation governing clinical studies is set to take effect in May-leaving interested parties only six more months to prepare for its arrival.
Applied Clinical Trials
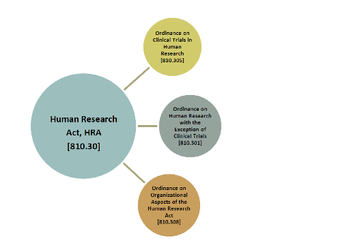
The country’s introduction of a new legal framework has it in sync with European law on clinical research.
Applied Clinical Trials
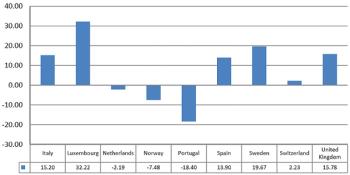
Examining why newly registered trials have fallen in Central and Eastern Europe, relative to other regions.
Applied Clinical Trials
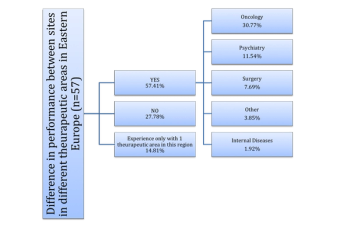
Survey sheds light on the regional-specific risks of managing clinical trial sites in Eastern Europe.

Applied Clinical Trials
Study uncovers subtle distinctions in attitudes and perceptions among the two groups.

Applied Clinical Trials
These three areas will be at center of drug development discussions in the year ahead.

Applied Clinical Trials
Regulatory: Assessing Study Drop in CEE Swiss Syncs Up Regulations Sites: Regional Risks in Eastern Europe Also in this issue: Orphan Drug Hurdles in Europe Patient Engagement: Illness-Level Impact Clinical Trial Technology in 2025

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials December 2015/January 2016 issue in an interactive PDF format.