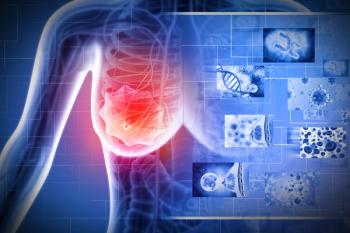
Enhertu achieved statistically significant and clinically meaningful improvements in progression-free survival compared with standard-of-care chemotherapy among patients with HR-positive, HER2-low metastatic breast cancer.

Enhertu achieved statistically significant and clinically meaningful improvements in progression-free survival compared with standard-of-care chemotherapy among patients with HR-positive, HER2-low metastatic breast cancer.

Although executive enthusiasm for decentralization of clinical trials has grown significantly, operationalizing these models requires meticulous planning and risk-based quality management.
Results from new analysis show RYBREVANT plus lazertinib consistently and significantly improved progression-free survival compared to osimertinib.

Under the new extension, FDA and CluePoints will enhance CluePoints’ SMART software to address a broader range of regulatory concerns.
Results from the Phase III CheckMate -9DW trial show Opdivo plus Yervoy produced a statistically significant and clinically meaningful improvement in overall survival compared with investigator’s choice of sorafenib or lenvatinib in the first-line treatment of unresectable hepatocellular carcinoma.

In the third and final part of this video interview, Sam Liu, VP of marketing, Vivalink looks forward in the BYOD space and forecasts potential advancements for consumer devices.

Results showed non-inferior efficacy and pharmacokinetics for subcutaneous amivantamab combined with lazertinib compared to intravenous administration.
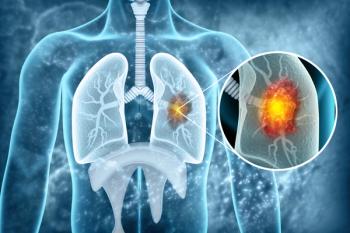
Tagrisso (osimertinib; AstraZeneca) reduced the risk of disease progression or death by 84% in patients with unresectable, stage III epidermal growth factor receptor-mutated (non-small cell lung cancer with tumors that harbor exon 19 deletions or exon 21 (L858R) mutations.

How artificial intelligence can aid in selecting patients, leveraging safety insights, and optimizing combination therapies.

In part 2 of this video interview, Sam Liu, VP of marketing, Vivalink discusses the key differences between consumer- and medical-grade wearables and their uses in clinical settings.
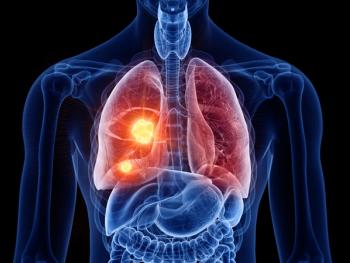
Results of the Phase III CheckMate -77T, CheckMate -816, and CheckMate -9LA trials show the potential of Opdivo to improve survival among patients with early and advanced stages of lung cancer.

New solution, CARA AI, will aid in the exploration of real-world data and leverage workflows such as imaging and cohort generation.
Approval of Breyanzi (lisocabtagene maraleucel) to treat adults with relapsed or refractory mantle cell lymphoma was based on findings from the open-label, multicenter, pivotal TRANSCEND NHL 001 trial.

Dupixent (dupilumab) was previously granted priority review status as an add-on maintenance treatment for certain adults with uncontrolled chronic obstructive pulmonary disease.

Three key considerations for deploying technology to help increase diversity in trials.

In part 1 of this video interview, Sam Liu, VP of marketing, Vivalink touches on concerns with data accuracy and security in bring-your-own-device models.
Patients with locally advanced or metastatic non-small cell lung cancer who were previously administered at least one line of therapy showed superior overall survival compared to docetaxel.

There is significant potential in decentralized models and digital health technology to expand diversity in clinical trials.

Lexicon will utilize Medidata’s decentralized clinical trial solutions to accelerate study of LX9211 in diabetic peripheral neuropathic pain.
Sarclisa (isatuximab-irfc) could become the first anti-CD38 therapy indicated in combination with bortezomib, lenalidomide, and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for a transplant.
Results showed median progression-free survival reached 12.6 months in patients with CLDN18.2 high or medium expression, including in patients with PD-L1 CPS<5.

Six trends to help guide trial master file strategy.

Keytruda (pembrolizumab; Merck) plus chemotherapy administered as neoadjuvant treatment and then as monotherapy postsurgery produced a statistically significant improvement in overall survival in patients with high-risk early-stage triple-negative breast cancer.

Wyatt discusses how these devices are changing patient monitoring in clinical trials.

Biotechs can successfully overcome bottlenecks in time-to-market for new drugs by embracing contracting innovations with the same passion applied to research breakthroughs.

New collaboration offers end-to-end solution, supplying and training sites on ophthalmic medical imaging equipment.
Results of the trial found that monotherapy led to clinical benefit in 16.7% of patients, including complete and partial responses in renal cell carcinoma (RCC) patients.

Expanded collaboration seeks to develop first-in-class treatments for cancer and cardio-renal-metabolic diseases.

Study results showed a 36% and 25% reduction in loss of asthma control (LOAC) with high and low doses of rilzabrutinib.

New agreement leverages Beaconcure’s technology to streamline review processes.