
CRAs now have to monitor sites? compliance with FDA?s Electronic Record, Electronic Signatures regulation.

CRAs now have to monitor sites? compliance with FDA?s Electronic Record, Electronic Signatures regulation.

When you're not at the conference center or your hotel, the city offers a wide variety of things to see and do.

Here's a back stage peek at the 39th annual DIA meeting and its organizers.

Things To Do and See in San Antonio

All that sightseeing can work up an appetite, but you might not understand the menus without this guide.

The study protocol and a set of specifications are the road map for developing the software and the support structures for an EPD study.

John Vogel, clinical researcher turned consultant, is passionate about outsourcing. He believes that pharmaceutical companies can gain competitive advantages through drug development outsourcing.

The clinical and technical teams use the protocol and a set of specifications as a road map for developing the software and the support structures for an EPD study.

Kimberly Irvine and Eileen Hilton (CenterWatch, Boston, MA, 2003), 158 pages, paperback, ISBN: 1930624395, $39.99.

Planning and preparation, along with imaginative innovations, can put an investigative site on the road to best research practices.

FDA surprises the pharmaceutical industry with its new interpretation of the Electronic Records, Electronic Signatures rule.

Economic realities are changing the way companies do business. One significant change is the growing numbers of freelancers in the clinical trials arena.

John I. Gallin, Ed. (Academic Press, San Diego, CA, 2002), 490 pages, hardcover, ISBN: 0122740653, $99.95.

HIPAA?s new Privacy Rule is in effect, and sponsors and clinical research professionals are learning how to apply it.

ACT's 11th annual guide highlights applications, software, Web-based solutions, and more.
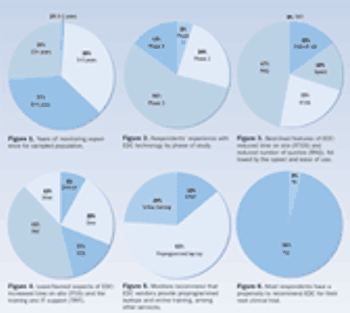
For years, EDC providers have claimed that the technology can increase efficiency and potential cost savings. Survey results indicate that clinical monitors agree.

Postmarketing research is conducted differently than preapproval research, but data gained using different standards can be difficult to integrate.

Readers respond to feature article and editorials.
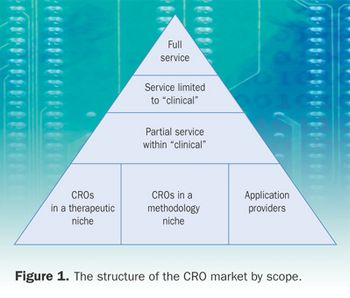
More than 1000 contract research organizations exist worldwide, and the number is growing. CROs differ widely in size and profile. Before a project can be contracted out, sponsors often spend valuable time searching for the CRO that best fits the project's specifications. Consultants are often needed to help hew a path through this jungle, a task made all the more difficult by the intentional withholding of information that would help sponsors decide. The World Wide Web would be an excellent means for CROs to communicate relevant and fluid information about their companies, and herein I offer a model for CROs to use.

For years, clinical trials have been largely sheltered from the liability lawsuit crisis that has plagued other industries. That is changing.
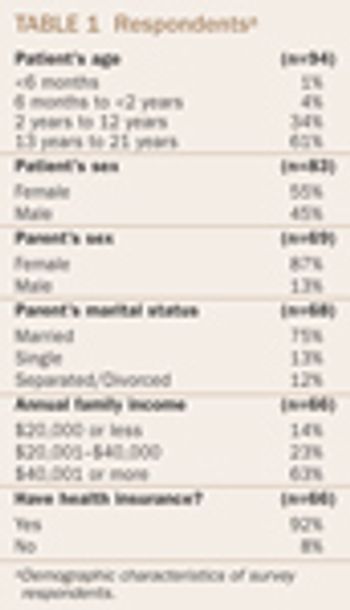
Recruiting children to participate in clinical drug trials is an activity closely scrutinized by regulators, bioethicists, ethics committees, institutional review boards, and the popular press. The FDA acknowledges the vulnerability of pediatric subjects and has implemented heightened regulatory safeguards. Pediatric clinical trials represent a powerful, emotional platform because of the potential for conflict of interest among a number of parties, including pharmaceutical company sponsors, study investigators, and parents.
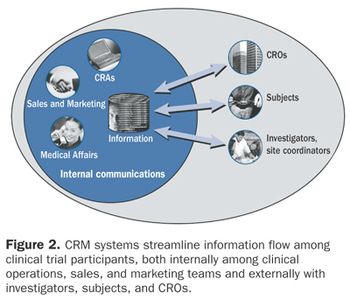
As the number and complexity of clinical trials grows-lengthening clinical trial durations and raising costs-sponsors and CROs need a way to streamline trial management, shorten study durations, and reduce expenditures. Customer relationship management (CRM) strategies update the paper-intensive methods of the past and offer an innovative approach to managing clinical trials-an approach that focuses on strengthening relationships between trial participants, especially investigators and subjects.

If it?s not clear who?s in charge, then no one is?and process improvement can be undermined.

Electronic data capture can be invaluable to speed clinical development processes. A recent study demonstrates the reliability of data collected by this method.

Security issues for electronic data capture may seem daunting, but they can become manageable with the right professionals and careful evaluation.
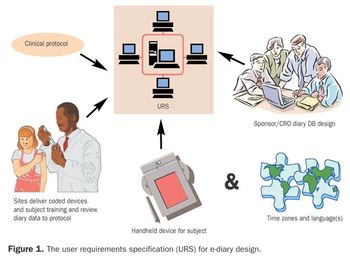
Subject diaries are used in about one-fourth of all clinical trials to collect data on primary and secondary endpoints.1 Part 1 of this four-part series on developing and implementing subject diaries compared and contrasted traditional paper and electronic diaries.2

Like all new technologies, the potential benefits of Linux also carry risk for pharmaceutical developers.

Registered dietitians can be effective study coordinators, as was shown during a hemodialysis study.

A review of clinical trials lawsuit king Alan Milstein's Web site.

With the rapid geographical expansion of clinical trials, industry professionals are increasingly willing to set up studies in exotic locations. Although their initial concerns about the quality of data from far-flung studies have dissipated, debate continues about the ethics of performing trials in new locations, especially in developing nations.