
Pharmaceutical research and development costs are growing faster than the market.1 This trend is important, because pharmaceutical companies finance R&D from a relatively fixed percentage (10%?20%) of global revenues.

Pharmaceutical research and development costs are growing faster than the market.1 This trend is important, because pharmaceutical companies finance R&D from a relatively fixed percentage (10%?20%) of global revenues.
The process of conducting clinical trials with plasma-derived products is essentially similar to that for any pharmaceutical product. However, trials with these products do present some different challenges in terms of trial design, patient recruitment, and clinical trial supplies.

Power source Raymond Hecker explains what's required to be 21 CFR 11 compliant, power-wise.

A dictionary of words and phrases whcih are crucial to the world of clinical trials.

Somewhere in a closed conference room, the IT wizards are plotting the data strategy of your company or institution. They are wrestling with the vast amount of raw data that is being collected and the challenges of making that data available as information to help in making decisions.

A full listing of college and university courses, training classes, and other educational opportunities.

These names, numbers, and titles are a phone directory for the regulatory world.

A listing of the alphabet soup of acronyms, abbreviations, and initials in the clinical trial world.

Find out about the purposes, rates, meetings, and publications of over two dozen socieites and associations.

Informed consent is a process, not just a form signed by prospective study subjects. Documents such as the Code of Federal Regulations describe the elements of informed consent, but lack substantive direction on the process of obtaining consent.1-2 The purpose of this article is to provide guidelines for obtaining informed consent. This guide can be useful to anyone involved in clinical research, particularly newcomers to the industry.

The rapid evolution of the biopharmaceutical industry has lead more and more companies to focus on the clinical development of their drug candidates, thus presenting the challenge of selecting the optimal strategy for conducting their clinical programs. Typically, biopharmaceutical companies have had three options: out-licensing their product, setting up their own clinical development operations, or outsourcing the clinical development to contract research organizations (CROs).

Moving ineffective personnel out of critical positions can be a difficult but necessary management task.

Clinical trials sponsors seek quick subject enrollment and high data quality, expressed by both strict adherence to good clinical practice (GCP) requirements and completeness and correctness of the data collected from investigative sites. However, the most informative sources of detailed information on data quality such as site monitoring visit reports, sponsor, and CRO audit reports are maintained as strictly confidential documents and are not publicly disseminated. Therefore, a substantial proportion of the information on data quality in clinical research that is available to the general public is based on anecdotal reports rather than well-referenced and organized observations. The U.S. Food and Drug Administration found no evidence of poor GCP compliance during inspections in the emerging clinical research countries, including Eastern Europe and the former Soviet Union.1-2

ACT 2003 Winter Forum Conference Programme
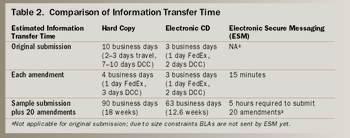
The expense of developing electronic submissions is manageable, even in a small company, and the benefits are great for both FDA and industry.
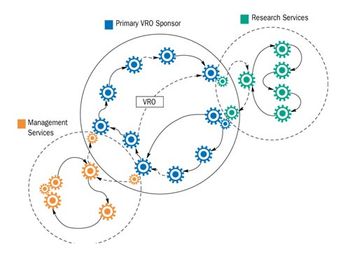
The economic consequences of inefficient work processes in clinical trials are significant. The average daily cost of drug development runs about $30,000 per day and rises by 10% to 12% per year. Development cycle times range from three to 12 years.1 In typical operational practice, the line management of a competitive firm strives to achieve the highest volume of successful new drug application (NDA) submissions (effectiveness) in the shortest practical time (efficiency). This combination reflects the business throughput of an organization.

eClinical Trials: Planning & Implementation is a useful resource for those new to the industry as well as practitioners already involved in planning and implementing eClinical trials.

Earlier this year, Applied Clinical Trials published "Clinical Monitoring: Answers to Questions about Good Clinical Practice," another excerpt from the book, Good Clinical Practice: A Question & Answer Reference Guide. Readers are referred to the July 2003 issue (pages 27-29) to read the full text of the reference guide introduction.

Relatively few people are familiar with the software design process, but many have had their homes remodeled; the two processes are more similar than dissimilar.

Interactive voice response systems are commonly used in clinical trials to manage the flow of trial medication supplies to sites and to manage the allocation of these supplies to individual subjects. Other advantages and uses include access to real-time information for trial managers, collection of diary card data directly from subjects, and as an aid to subject recruitment.1

Both the pharmaceutical industry and regulatory authorities worldwide have recognized the need for more clinical studies in children. A recent European conference addressed the issues.

It is increasingly important for clinical trial personnel to break through the obstacles that language and culture present when conducting trials away from their home countries.

Restrictive regulations and cultural differences make regulatory approval in Japan elusive, but adopting new strategies may make that lucrative pharmaceutical market more accessible to non-Japanese companies.

Application, software, Web-based solutions, and more.

Interdepartmental politics can suffer from competition and mistrust, but for clinical trials to be truly efficient these obstacles need to be replaced by respect and purpose.

Information Showcase

Now that FDA is enforcing its Electronic Records, Electronic Signatures rule many pharmaceutical companies are reconsidering their data systems.

Bayesian isn?t the most popular statistical method yet, but it is gaining ground in a number of clinical trials areas.
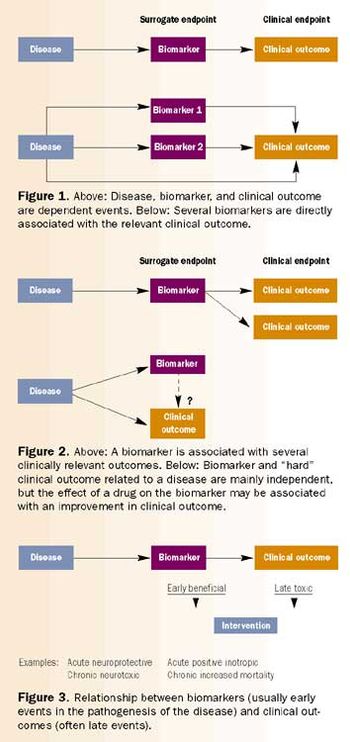
Both large and small pharmaceutical companies have learned that the value of their development candidates increases once clinical research has demonstrated their proof of concept. Sponsor companies face the challenge of moving new blockbuster drugs to market as rapidly as possible.

A newly published reference guide answers a wide variety of GCP questions.