
Digital dashboards will allow you to pilot your clinical trial from the captain's chair.

Digital dashboards will allow you to pilot your clinical trial from the captain's chair.

Digital dashboards will allow you to pilot your clinical trial from the captain's chair.

Vocel's mDiary, Clinical Systems, Inc.'s ClinBook, and Provisio Inc.'s iTrials

Find out all you need to know about the District of Columbia to impress colleagues and clients.

Welcome to ACT's Washington DC Exhibitor Profiles Supplement for 2004.

A United Nations of taste buds is a mere walk or short cab ride from your hotel. Here are some highlights to whet your appetite.

A map of the area and some welcoming words.

DC day spas for the ultimate in relaxation.

With so many enjoyable and educational opportunities at hand, keeping your spouse and children occupied is an easy task. The dilemma is deciding what to do first!

In contract research and contract bridge, the cards aren't as important as the people holding them.

To better understand the complex world of licensing and enterprise software, consider a driving analogy.
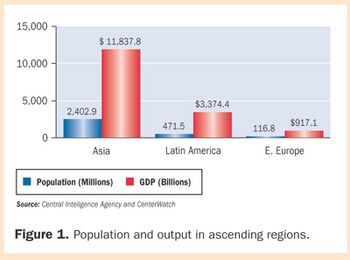
Clinical trials in developing nations-called "ascending markets"-are exploding. These regions offer large naïve subject populations, low operating costs, and increasingly stable testing infrastructures, but regulatory reform is really driving the growth. CenterWatch data show 20%?30% of clinical trials are being conducted in ascending regions.
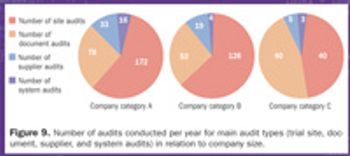
An extended survey was conducted on the topic of clinical quality assurance (CQA) benchmarking in pharmaceutical companies to gather new data.

Datatrak International's Phase IV, Espicom's World Pharmaceutical Markets, and Keris, Inc.'s Vital Consent

The Uppsala Monitoring Center's WHO Drug Dictionary, Information Mediary's Med-ic Smart Package, and Relsys International Inc.'s Argus J

New requirements for clinical research teams, and administrative responsibilities and constraints for investigators conducting noncommercial trials.

Daniel Callahan (University of California Press, Berkeley, CA, 2003), 341 pages, hardcover, ISBN: 0-520-22771-9. $29.95.

Readers respond to articles and commentaries.

IntraLinks' IntraLinks, Pharsight Corp.'s Trial Simulator, and Thomson CenterWatch's Drugs in Clinical Trials Database and eDirectory of the Clinical Trials Industry

May 2004 Issue Advertising Opportunity

ACRP Conference Promotional Opportunity

ACTion Newsletter logo
Art / Image Article

Medidata Solutions's Vision Developer, Gentiae Clinical Research, Inc.'s LifeSignals, and Integic Corp.'s CRF WorkManager

Arbitrarily scheduled meetings eat away at valuable time otherwise spent actually getting things done.

When FDA commenced discussions on the use of electronic records in lieu of paper records in 1991, it embarked upon a six-year consultation exercise culminating in the issuance of the final rule "21 CFR 11,"1 which came into force on 20 August 1997. The final rule itself is succinct (approximately 2000 words), but has given rise to massive amounts of commentary and interpretative text. The latest guidance aims to address issues with these secondary texts rather than the rule itself.
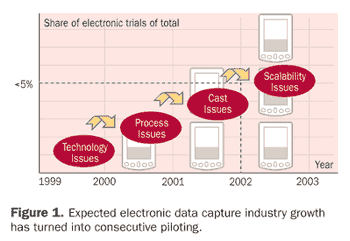
As so many articles have noted,1 the uptake of electronic data capture (EDC) and associated technologies has continued to be disappointing. Even though 20 years ago experts predicted that electronic case report forms (CRFs) would replace paper as a natural consequence of the introduction of computers, this has not been the case.

Disease evaluation by imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) is an accurate, reproducible, and easily accessible methodology used in pharmaceutical trials.

Three profiled companies have three different needs when it comes to choosing PDA.

Two experts share their wisdom on how to correcly apply for Medicare coverage for clinical trials.