
2005 ACT Directory & Buyers Guide

Have your subjects record electronic information in blue ink with Health Decisions' writing instrument.

Readers respond to articles and commentaries.

In the biomedical research industry, and at Pfizer in particular, we are committed to what we call "The Three As"-making medicines available, accessible, and affordable.

A new database design is behind a way of generating reports when you want them--such as right now

Pharmaceutical companies need to integrate pediatric assessments into the standard drug development process and build up pediatric competence.

Weyers, Wolfgang (New York: Ardor Schribendi, Ltd., 2003) 755 pages.

Indonesia, Sri Lanka, India, Thailand, Somalia, Burma, Malaysia, Maldives, Tanzania, Seychelles, Bangladesh, Kenya We wish to express our deepest sympathy to all those who grieve --Applied Clinical Trials
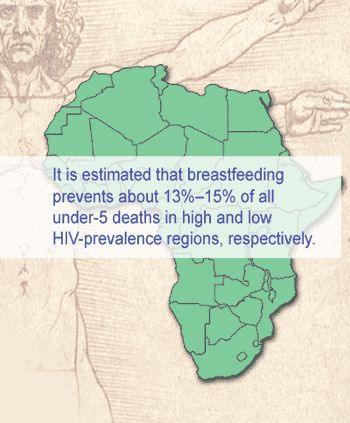
The data obtained by The Bellagio Child Survival Study Group has made the identification of priority research subjects more precise and relevant to developing countries.

The Pediatric Rule made everyone begin to assess, think, and plan for pediatric needs.

Think of Teranode's modeling software program as the world's most sophisticated blackboard

Following the examples of these six companies might change your organization for good.

Readers respond to articles and commentaries.

Stephen I. Ankier and Angus E. Donald (Ankier Associates, 2004), 213 pages, £145

Third Wave Technologies' Invader,? MDS Pharma Services' Centralized ECG Service,? and Covance's StudyTracker?

We work so hard to recruit subjects for clinical trials, so why don't we try harder to retain them?

Celine M. Clive (Interpharm/CRC Press, 2004), 288 pages with CD-ROM, hardcover, ISBN: 0849321816, $229.95

November 2004 Advertising Opportunities

invivodata's SitePRO,? QuickSTAT's QuickOnlineRx,? and Cold Chain Technologies' KoolWatch?

December 2004 Advertising Opportunities!

A new reference guide clarifies uncertainty surrounding this sometimes misunderstood document.
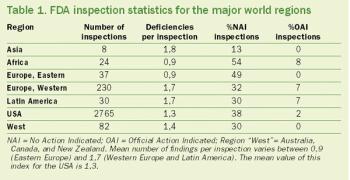
The first U.S. investigational new drug (IND) clinical trials outside of the United States were conducted in Europe in the late 1970s. In the early 1990s, Central and Eastern Europe (CEE), Latin America, Asia, and selected countries on the African continent (mostly South Africa) became involved in clinical trials.
Interspond’s RxPaging, Scientific Software Tools, Inc.’s VISTA, and Jurilab’s DrugMEt.

October 2004 Supplement Advertising Opportunities

Move away from the "service" model if it means being subservient.

Readers respond to articles and columnists.
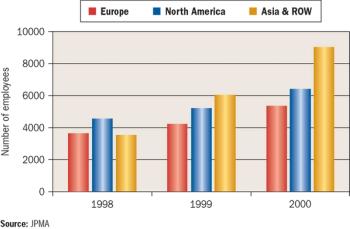
Long overlooked as global drug innovators, Japanese pharmaceutical companies are gaining attention for their novel clinical development programs.

Arthur L. Caplan, Ph.D., chair of the University of Pennsylvania's Department of Medical Ethics, expressed his frank views about issues that are ailing the drug industry today during his DIA Keynote Address in mid-June.

September 2004 Advertising Opportunities!