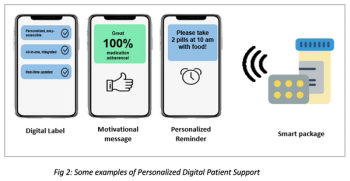
What the next generation medication management in clinical trials should entail and insights on smart technologies and synergistic partnerships—backed by real-life implementation use cases.

What the next generation medication management in clinical trials should entail and insights on smart technologies and synergistic partnerships—backed by real-life implementation use cases.

Digitalization has revolutionized an industry once reliant on paper collection.

Capacity management and stability offer value for clients, their employees and FSP vendors.
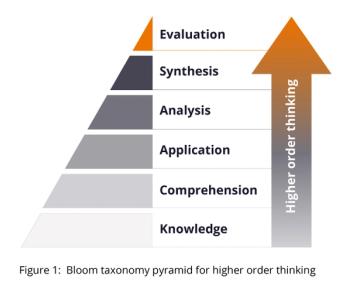
Developing a higher level of critical thinking can create a comprehensive risk story and properly direct mitigations throughout your organization.

Dr. Hanne Bak, Senior Vice President of Preclinical Manufacturing and Process Development at Regeneron speaks about her role at the company as well as their work with monoclonal antibodies, the regulatory side of manufacturing, and more.

A focus on the position of law for e-signatures, their benefits, and the role of Clinical Research Malaysia in educating the industry on the practicality of e-signature.
How to assess common obstacles for opportunity and create more agile and adaptive clinical trials.
A summary of how organizations approached remote operating models and the experiences and effectiveness of clinical research professionals in their roles and responsibilities during the COVID-19 pandemic.

COVID-19 creates unique challenges for medical monitors.

How it has taken a global pandemic to reinforce the intrinsic benefits.

COVID-19 reshaping the roles of CRAs overseas as industry shifts to remote monitoring.

There's no one-size-fits-all approach during the COVID-19 pandemic

Behind-the-scenes look at vaccine development.

Compression between phases to accelerate cycle time.

Assessing the opportunities, considerations and implications of decentralized trials—and why they’re here to stay

The pandemic spurred an urgency to innovate that improved clinical development in ways that should benefit the industry beyond COVID-19.

Identifying the ‘significant six’ areas of clinical study conduct to mitigate risk.

Fully-integrated, component-based CDMS offers flexibility, customization, and efficiency.

Applying a year's worth of lessons to next generation clinical trials.

A top-down analysis of the decentralized trial model’s benefits and impact on the industry.
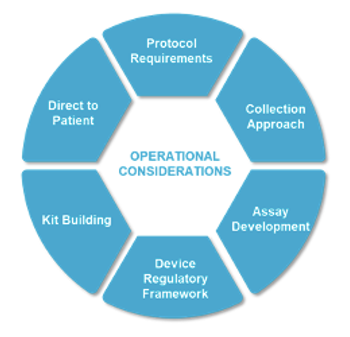
Operational and patient burden considerations for self-collection of blood specimens in clinical trials

Life sciences companies challenged by need to minimize health risks without sacrificing data quality during pandemic.

Non-profit biotech organization Cure Rare Disease utilizes collaboration amidst COVID-19 pandemic to catalyze speed of therapeutic research.

COVID-19 speeds up clinical research and creates new advancements within industry.

Clinical operations professionals, burdened by lack of data standardization, turn to technology in hopes of streamlining regulatory processes for the future.