Phase III REMIX-1 and REMIX-2 trials demonstrated that remibrutinib, an oral BTK inhibitor, significantly reduced chronic spontaneous urticaria symptoms in patients unresponsive to second-generation H1-antihistamines.

Phase I Trial Supports Development of Once-Yearly Lenacapavir as Long-Acting HIV PrEP Option
Pooled Clinical Trial Meta-Analysis Shows Superior Response Rates with Padcev Plus Keytruda Treating Metastatic Urothelial Carcinoma

Phase III REMIX-1 and REMIX-2 trials demonstrated that remibrutinib, an oral BTK inhibitor, significantly reduced chronic spontaneous urticaria symptoms in patients unresponsive to second-generation H1-antihistamines.
Analysis shows the addition of Keytruda (pembrolizumab) to standard chemoradiotherapy for newly diagnosed, locally advanced cervical cancer is not cost-effective at its current price.

Phase III WAYPOINT trial shows Tezspire (tezepelumab) significantly reduces nasal polyp size and congestion severity in patients with severe, uncontrolled chronic rhinosinusitis with nasal polyps, while also decreasing the need for surgery and systemic glucocorticoids.

Xolair (omalizumab) shows superior efficacy and safety compared to multi-allergen oral immunotherapy in treating food allergies in the Phase III OUtMATCH trial, suggesting the potential to facilitate the introduction of allergenic foods into patients' diets after treatment.
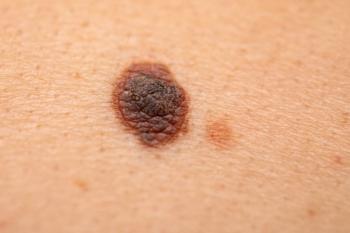
Results from the RELATIVITY-098 trial of Opdualag in the adjuvant treatment of completely resected stage III-IV melanoma did not meet the primary endpoint of recurrence-free survival.
Phase III PRESTIGE-AF trial shows that direct oral anticoagulants significantly lower the risk of ischemic stroke in survivors of intracerebral hemorrhage (ICH) with atrial fibrillation, but increased the risk of recurrent ICH and major bleeding complications.
The Phase III GEMSTONE-303 trial demonstrated that sugemalimab plus chemotherapy significantly improves overall survival and progression-free survival compared to chemotherapy in patients with locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma with PD-L1 CPS ≥5.
The combination of Opdivo plus Yervoy produced significant Phase III trial results in the first-line treatment of patients aged 12 and older with unresectable or metastatic MSI-H/dMMR colorectal cancer.

Data from the Phase III EMERGE trial show treatment with Symbravo provided more rapid and sustained migraine relief with improved quality of life in patients who previously had an inadequate response to CGRP treatments.

Tremfya could become the first IL-23 inhibitor with fully subcutaneous induction and maintenance options in the treatment of moderately to severely active ulcerative colitis.
The first prespecified interim analysis of the Phase III AMPLIFY trial found that fixed-duration Calquence (acalabrutinib) plus venetoclax, with or without Gazyva (obinutuzumab), significantly improved progression-free survival compared to chemoimmunotherapy in fit patients with previously untreated chronic lymphocytic leukemia.
The final analysis of the Phase III CheckMate -816 trial confirmed a statistically significant overall survival benefit for patients with resectable non-small cell lung cancer treated with neoadjuvant Opdivo (nivolumab) plus chemotherapy.
Five-year data from the POETYK PSO long-term extension trial confirm the sustained efficacy and safety of Sotyktu (deucravacitinib) for moderate-to-severe plaque psoriasis, with patients maintaining high clinical response rates and no new safety concerns identified.
Updated data from the Phase III TALAPRO-2 trial show that the combination of Talzenna (talazoparib) and Xtandi (enzalutamide) significantly improves overall survival in patients with metastatic castration-resistant prostate cancer regardless of mutation status.
The Phase II eNRGy trial showed durable antitumor activity with zenocutuzumab (Bizengri) in patients with NRG1 fusion–positive cancers, particularly non-small cell lung cancer and pancreatic cancer.
Phase III EV-302/KEYNOTE-A39 trial confirms that the combination of Padcev (enfortumab vedotin) and Keytruda (pembrolizumab) provides sustained and significant overall survival and progression-free survival benefits compared to chemotherapy in previously untreated patients with locally advanced or metastatic urothelial cancer.
Gazyva/Gazyvaro (obinutuzumab) was found to significantly improve complete renal response in lupus nephritis patients, reinforcing its potential as an effective treatment option while also allowing for a reduction in corticosteroid use.
In the ALEXANDRA/IMpassion030 trial, Tecentriq (atezolizumab) added to postoperative chemotherapy was not found to improve treatment outcomes for patients with high-risk early-stage triple-negative breast cancer.
Braftovi (encorafenib) plus Erbitux (cetuximab) and mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) improved progression-free survival and overall survival compared to chemotherapy in patients with metastatic colorectal cancer with a BRAF V600E mutation.

The 12-month open-label extension of the PACIFIC trial demonstrated that bexicaserin significantly reduced seizure frequency in patients with developmental and epileptic encephalopathies while maintaining a favorable safety and tolerability profile.
The Phase III HYPERION trial was stopped early after strong positive interim results from the ZENITH trial demonstrated the efficacy of Winrevair (sotatercept-csrk) in treating pulmonary arterial hypertension, making it unethical to continue.
Abelacimab was found to significantly lower factor XI levels and bleeding events compared to rivaroxaban (Xarelto) in patients with atrial fibrillation at moderate-to-high risk for stroke.
The Phase III INAVO120 trial found that a combination of Itovebi (inavolisib) with Ibrance (palbociclib) and Faslodex (fulvestrant) significantly improved overall survival and progression-free survival in patients with PIK3CA-mutated, HR-positive, HER2-negative, endocrine-resistant advanced or metastatic breast cancer.
Phase III CheckMate -8HW trial shows the combination of Opdivo (nivolumab) and Yervoy (ipilimumab) significantly improved progression-free survival and overall response rates in patients with microsatellite-instability–high or mismatch-repair–deficient metastatic colorectal cancer.
Keytruda (pembrolizumab) plus Lenvima (lenvatinib) and chemotherapy improved progression-free survival but did not achieve statistical significance for overall survival in patients with advanced HER2-negative gastroesophageal adenocarcinoma.