
Series Part 1—Introduction and the relationship between QTL and KRI.


Series Part 1—Introduction and the relationship between QTL and KRI.

With large shift to decentralized strategies, industry roles appear set for change.

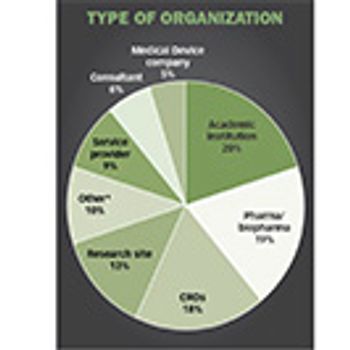
The perfect storm of industry growth, opportunity, and demand is translating to solid salary and satisfaction levels among clinical trial professionals, according to new survey.

Reference tool is a Rosetta Stone for clinical research. A look at the updates and enhancements in latest version.

The Clinical Endpoints Adjudication Group and Ethical Clinical designed and implemented an industry survey to evaluate factors that drive the use of endpoint adjudication in clinical trials.

The standardization of trial metrics offers numerous benefits towards overseeing clinical trials, optimizing clinical operations and mitigating study risks. However, on a situational basis customized metrics may become necessary to address study-specific risks, uncover unknown issues and demonstrate results.

Due to mistakes by research professionals, clinical trials have been subject to delays or even cancellations. The National Board of Medical Examiners is working to reverse this trend by introducing an assessment to test the knowledge of clinical researchers.

New study finds that most biopharma companies are now using a standard set of key performance indicators.

Analysis from multiple reports shows that Phase I clinical trials typically have a low rate of success. The lowest success rate occurs during Phase II of research, while those who make it to Phase III do not fare much better.

Over a year ago, Claravant started the research and vetting required to “handicap” the risk of a drug being approved by FDA, and ultimately becoming commercialized in the form of a ratings system.

The clinical trials industry can benefit from site-driven metrics for process improvement and site scoring.