Developing a higher level of critical thinking can create a comprehensive risk story and properly direct mitigations throughout your organization.

Developing a higher level of critical thinking can create a comprehensive risk story and properly direct mitigations throughout your organization.

Identifying the ‘significant six’ areas of clinical study conduct to mitigate risk.

Researchers aren't taking full advantage of advances in trial technologies. A lack of clarity in the current version of the International Council for Harmonization "Guidelines for Good Clinical Practice", could be the problem.

Exploring effective strategies for sponsors and CROs to ensure both their CRAs and sites are supported for high levels of site acceptance and streamlined remote monitoring.

Organizations implementing RBM continue to struggle with a number of questions regarding the relative contributions to quality of on-site monitoring, centralized statistical monitoring, and clinical data management reviews—and what role each activity should play.

CEO of MANA UBM, Penelope K. Manasco, explores the different approaches to determine what the right 'easy button' is to push to achieve effective clinical trial conduct and oversight.

The biopharmaceutical industry must establish customized approaches to managing Quality Management Systems within their clinical trials processes.

Centralized monitoring is the core of RBM and helps study teams decide on on-site monitoring activities and site intervention. Thus, the right mix of KRIs and Central Statistical Monitoring Reports is crucial for trial success.


The pharma industry needs an improved risk-based management approach to better handle the increased complexity of trials, to improve the quality of studies and to better adhere to new guidelines from regulatory agencies.

The ICH is publishing its long-awaited guidelines on how to conduct clinical monitoring in trial management. Sponsors and CROs can integrate existing and emerging technologies while transforming their reactive oversight strategies to an RBM approach using these steps.

Risk-based monitoring continues to remain in the spotlight as an accepted and oft implemented approach that is likely to become an industry standard. However, concerns have mounted about the potential impact of these changes on clinical study sites.

Investigational sites’ readiness to support new monitoring initiatives such as risk-based monitoring and centralized monitoring will dictate their success. Here are key aspects that sponsors and CROs should establish with investigational sites while implementing these new initiatives.

This 3-part series provides insight into ensuring compliance with the ICH E6 (R2) Addendum to take effect later this year. Part 3 covers the value of embracing a centralized, technology driven approach to risk based monitoring.

Many have inquired about writing an RBM plan due to recent changes in industry execution and an increase in RBM experiences. Moe Alsumidaie responds with a brief methodology on developing a risk management process to create an RBM plan.

A risk-based approach to quality trial oversight, adopted by the FDA and EMA, has resulted in an array of new technology solutions being released.

RBM and changes in quality management are impacting study teams, CRAs and sites.

With the introduction centralized monitoring in a risk-based monitoring (RBM) approach, the central monitor is emerging as a key and important role.

Good clinical trial risk management involves good protocol design and study operational design at the sites.

Using the five steps to a risk management plan from other industries--Identify, Assess, Plan, Track and Control--to inform a RBM approach in clinical trials.

Infinity Pharmaceuticals will use Medidata's RBM solution in an upcoming Phase III oncology study for patients with indolent non-Hodgkin lymphoma.
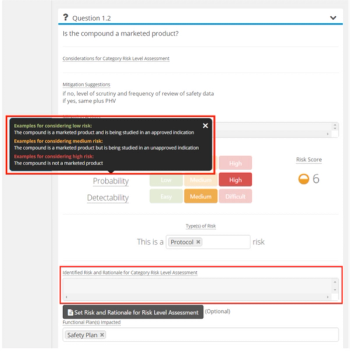
TransCelerate’s Risk Assessment and Categorization Tool (RACT) has exhibited usefulness with study teams and has guided organizations, such as Cancer Research UK (CRUK) to adopt their own RBM questionnaires


CBI’s conference proves RBM was just the beginning. Now companies are honing in on the risks and mitigations related to clinical trial data reporting.

The ability to have access to real-time information through leveraging technology combined with a strategic business perspective allows companies to implement a true quality management system.