
CluePoints, a provider of Centralized Statistical Monitoring (CSM) solutions for clinical trials, introduced software to enable analyses of operational data using a risk-based monitoring approach.

CluePoints, a provider of Centralized Statistical Monitoring (CSM) solutions for clinical trials, introduced software to enable analyses of operational data using a risk-based monitoring approach.

With the clinical trial industry’s focus on improving monitoring efficiency and leveraging a variety of risk-based monitoring (RbM) models, novel eClinical technologies are emerging to facilitate clinical trial monitoring productivity.
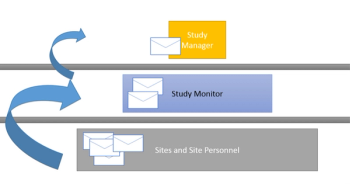
“Never assume that something obvious is true,”
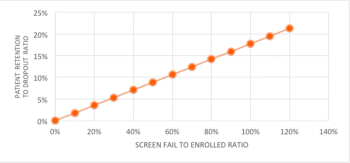
Clinical trial patient retention and dropout continues to be an issue amongst biopharmaceutical sponsors, as patient dropouts minimize the statistical power of clinical trial data, requiring study teams to enroll additional patients.

Often overlooked in early evaluation of RBM are the tools and technologies required for successful implementation. Without the right tools to track and prioritize the shift from traditional monitoring to RBM, successful implementation is doubtful, posing detrimental consequences not only for the monitors, but also the overall trial.

That's because the discussion shifted away from one focused on changing monitoring methods and doing reduced source document verification (SDV) to 'intelligent monitoring.'

The concept of risk-based monitoring (RbM) is evolving, as nonprofit organizations continue to collaborate with the biopharmaceutical/medical device industry to investigate, pilot and implement RbM practices.

Redefining this paradigm may help dispel current misperceptions around RBM and better deliver on the promise of this growing approach.

While capable of conducting high-quality trials, the current oversight process can be expensive and inefficient.

Steve Powell, PRA Executive VP Clinical Informatics and Late Phase Services Talks About Risk-Based Monitoring (RBM)
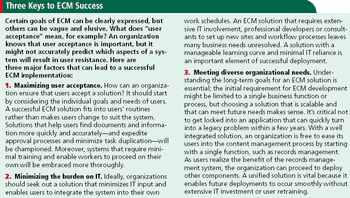
True efficiency requires the move to a company-wide ECM solution that supports the entire content lifecycle.