
Pearl IRB earned this distinction in December 2014 by demonstrating extensive safeguards in all levels of the research operations and abiding by high standards of excellence for all research.

Pearl IRB earned this distinction in December 2014 by demonstrating extensive safeguards in all levels of the research operations and abiding by high standards of excellence for all research.



CRF Health, a global provider of electronic Clinical Outcome Assessment solutions for the life sciences industry, announced a new partnership with Vodafone.


Theorem Clinical Research, a prominent global contract research organization, and Biomedical Systems, a leading provider of comprehensive centralized diagnostic services, have formed a strategic partnership

PHT next week will announce a new suite of clinical research patient engagement apps at the Summit for Clinical Ops Executives conference in Orlando


Leading Biopharma Company Adopts Medidata Technology to Bring Operational Efficiencies and Greater Speed to Innovative Clinical Study on Metabolic Disorders

PCI is pleased to announce that further to the acquisition in September 2014 of Biotec Services International, the company has rebranded as part of the PCI group.


Medidata, the leading global provider of cloud-based solutions for clinical research in life sciences, today announced a strategic collaboration with Garmin International Inc.


ClinPlan provides automated, efficient, and accurate way to assess and reassess trial expenses over time throughout the life of the trial.

Clinverse, Inc., provider of automated financial management technology solutions for clinical trials, announced today that it has expanded its suite of products with the addition of ClinPlan™, purpose-built software for clinical trial budget management and forecasting.


Accelovance, Inc. , a CRO therapeutically aligned in the areas of Oncology, Vaccines, and General Medicine; today announced it has acquired Altair Clinical, Ltd.


ICON, a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries, today announced it has agreed, subject to certain customary closing conditions, to acquire MediMedia Pharma Solutions for a cash consideration of $120 million.


ERT, a leading provider of technology solutions and services that increase the reliability and efficiency of high-quality patient data collection, today announced that its electronic Suicide Risk Assessment (SRA) system – AVERT® – will be implemented by Rutgers University

The Clinical Trials Transformation Initiative (CTTI) has announced recommendations to streamline Good Clinical Practice (GCP) training of investigators who participate in clinical trials.

TransCelerate BioPharma Inc. today announced two new members, Merck & Co. Inc., and Novo Nordisk, to the biopharmaceutical non-profit organization.

ArisGlobal, a leading provider of solutions to the life sciences industry, has announced that agDisclosure, its clinical trial disclosure solution, now supports Version 10 of the European Medicines Agencys EudraCT database

ArisGlobal, a leading provider of solutions to the life sciences industry, has announced that agDisclosure, its clinical trial disclosure solution, now supports Version 10 of the European Medicines Agencys EudraCT database

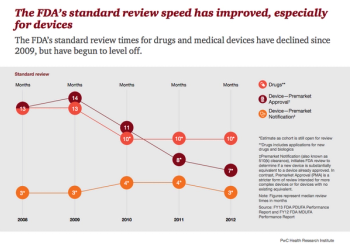
Consumers and drug and device manufacturers are changing practices and shifting attitudes toward the Food and Drug Administration (FDA). Increased pressures for speedy access to breakthrough drugs and medical devices, and a focus on value in addition to medical benefit, are driving these changes.

Improves data quality and regulatory compliance by combining scientific training with innovative electronic implementation of instruments

The pharmaceutical industry is further optimizing the collection of high quality electronic patient reported outcomes (ePROs) data for clinical research, with PHT Corporation Rater Training programs
