
A move back to in-house management of clinops could be on the horizon for sponsors.
Lisa Henderson is Editor-in-Chief of Applied Clinical Trials and Pharm Exec. She can be reached at lhenderson@mmhgroup.com.

A move back to in-house management of clinops could be on the horizon for sponsors.

Scott Gray, founder and CEO Clincierge, discusses the biggest changes in trial operations, patient centricity, and shares a few stories about obstacles overcome during the coronavirus pandemic.

Brad Power, Founder of CancerHacker Lab, speaks about his innovative crowdsourcing approach to help cancer patients, his own battle with lymphoma, and the unique challenges brought to the table by clinical trials in oncology.

One misclick by an employee can lead to a number of critical issues including clinical data breaches.

Dr. Hanne Bak, Senior Vice President of Preclinical Manufacturing and Process Development at Regeneron speaks about her role at the company as well as their work with monoclonal antibodies, the regulatory side of manufacturing, and more.

Mike Rea, CEO and Founder of IDEA Pharma, speaks about how his newly launched sister company—Protodigm—takes lessons learned from IDEA, combined with ideas borrowed from engineering and tech development, to form a new concept in pharma and biotech to innovate drug development with minimized risk and without bias.

Dr. Peter Schueler, Senior VP Drug Development Services, Neurosciences at ICON, talks about topics discussed in a timely new book he edited titled Innovation in Clinical Trial Methodologies.

John Reites, CEO of THREAD Research, discusses how his organization is adapting to decentralized trials and efforts they're taking to increase patient recruitment.

Dr. Theodore Leng, MD, Medical Advisor to Verana Health and Director of Clinical and Translational Research at the Byers Eye Institute at Stanford, Stanford University Medical School, talks about a recent study he led and presented in early May at the Association for Research in Vision and Ophthalmology Meeting.

A brief look at the market for mergers and acquisitions since the year of COVID.

Courtney Granville, Global Associate Director, Research and Scientific Programs at DIA, talks about the options decentralized trials provide, the options that DIA is offering its members to learn in a hybrid environment, and the options healthcare and research should provide for patients now.

Upcoming Clinical Trials Day allows industry to look back—and forward.

Behind-the-scenes look at vaccine development.

Applying a year's worth of lessons to next generation clinical trials.

A look forward to 2021 for the clinical trials industry.

Regulatory concerns remain despite industry's quick adaptation of decentralized trials.

The FDA hints that their increased speed in issuing guidances during the pandemic could become the new norm.

ACT's Editor-in-Chief, Lisa Henderson, assesses the sustainability of remote trials.

A look into the future based off what we've learned about trials during the pandemic.

While clinical trials are starting to make a return from COVID-19-related interruptions, cracks in their traditional operation are being exposed.

Recent surveys of the public and patients concerning COVID-19 highlight the larger issue around health and/or scientific literacy among the general public.

With the bevy of coronavirus-specific information, databases and trackers, registries, and other tools out there at the moment, the challenge in store is integrating these resources to help in both clinical care response and informing drug intervention in real-time.

Industry experts weigh in on how much traditional approaches in clinical operations need to change to meet new expectations for clinical delivery.

COVID-19 will not only leave a long legacy on public health, but it may also force companies that have been lagging behind the disruption curve to adopt technologies and processes that will make clinical trials more efficient.

A glimpse into the WCG webcast held to address the concerns about clinical trial conduct in the age of COVID-19.

The trends that seem to be garnering the most discussion today among clinical trial management and operations professionals.
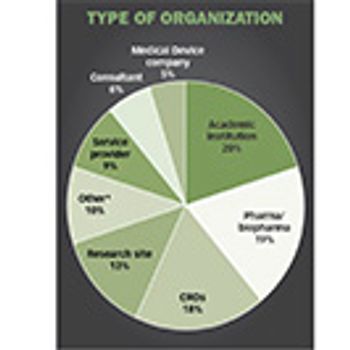
The perfect storm of industry growth, opportunity, and demand is translating to solid salary and satisfaction levels among clinical trial professionals, according to new survey.

Trial participants are willing to share data to improve their lives and the lives of others, but they also request that data and clinical trial results are shared back to them.

Some thoughts around the industry continuing to grapple with the high price tag associated with drug development costs.

While the future of the industry is still uncertain, a slew of uplifting ideas around innovation could help turn things around.