
New survey data, coupled with recent research, spotlight the current views and concerns around multi-regional studies.
Lisa Henderson is Editor-in-Chief of Applied Clinical Trials and Pharm Exec. She can be reached at lhenderson@mmhgroup.com.

New survey data, coupled with recent research, spotlight the current views and concerns around multi-regional studies.

Amid increased consolidation in the CRO space, more biopharma alliances, and the emergence of new clinical technology platforms, finding the formula to turn all these good intentions into tangible improvements in drug development practice remain elusive.

As our magazine embarks on the next 25 years of hopeful service covering the clinical trial enterprise, a quest for industry will be lessoning the complexity of drug development while remaining vigilant in protecting the patient.

Why following one consistent playbook for trial management should be at the core of outsourcing partnerships in drug development.

How CROs and sponsors can step up their relationship game.

Reaching the point of seamless integration of patient-care options won't be easy, but you can visualize how to get there through the prism of the "Clinical Trial of Tomorrow/"
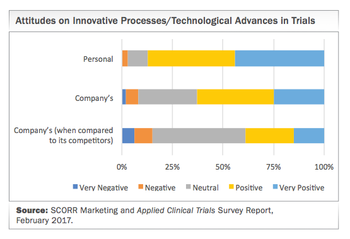
Highlighting the six broad technology themes poised to transform future R&D.
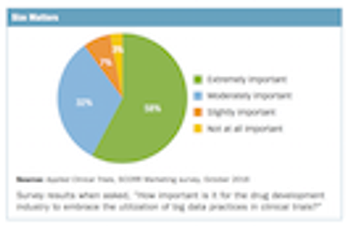
Survey collects thoughts from industry professionals on the current and future impact of big data in clinical trials.

A new group of collaborators has formed to determine the benefits and risk tradeoffs Parkinson’s disease patients are willing to make for a potential new therapy. Dr. Brett Hauber of RTI Health Solutions spoke to us about the collaborative.

Updated guidance incorporates the use of a patient-reported outcomes questionnaire-which could enable more endpoints and greater patient stratification for COPD trials.

Technology innovations have been introduced into the clinical trials space that have the ability to change the trial experience for all involved. Egg’s TRIAL 360 platform is no exception, as its goal is to provide a networking platform to keep investigators and study stakeholders engaged in the trial.

With the ever-present gap in ability to recruit patients into clinical trials, emerging solutions take different tactics to find the right patients and enroll them quickly.

The Avoca Group’s Quality Consortium’s fifth annual Summit took place in May and June of this year bringing together over 250 attendees from 50 companies. This years event brought out unifying themes around innovation and patient centricity.

More precision models in connecting patients to clinical trials and treatment options are vying to close the ever-elusive recruitment gap.

Survey delves deep into what constitutes true game-changing innovation in clinical development.

Continuous Glucose Monitoring (CGM) devices have the ability to replace the traditional finger-prick to measure glucose levels in a patient’s blood. Quintiles’ device expert, Sam Osman, explains CGM and how the FDA decision could affect clinical trials.

OmniComm’s Keith Howells speaks to ACT about whether electronic data capture (EDC) can be considered innovative in today's pharma.
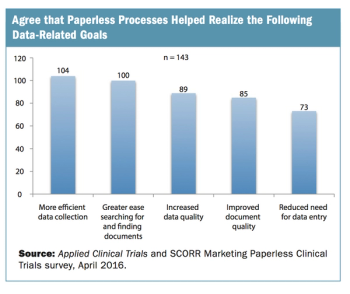
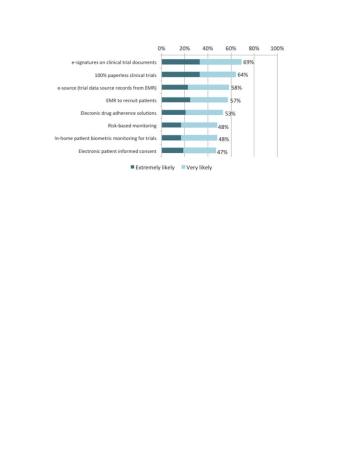
Survey reveals mixed adoption of paperless data collection in clinical trials, pointing to the need for greater alignment of new eClinical technologies and study conduct.

CDER official comments on the significance of ICH's new alternative path for identifying the cardiac safety issue of QT prolongation in non-cardiac drugs.

On Wednesday, DrugDev is debuting its Site Activation Module at the SCOPE Summit. The company believes the module will greatly improve all tasks of site activation, from protocol feasibility, site identification and selection, to contracts and the collection of site regulatory documents.

Changes to cardiac safety assessments could equal more drugs in the pipeline and cost savings to sponsors.
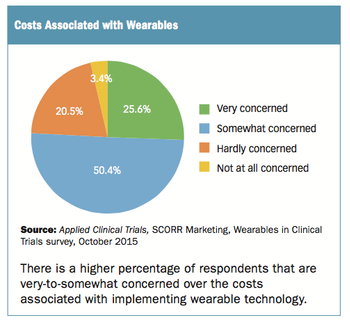
Survey spotlights the concern levels associated with the adoption of clinical trials using wearable technology.

French safety authority confirms what had been speculated--Phase I volunteers received the fifth of the highest dose escalation of the investigational drug at the same time, which goes against EMA recommendations.

With global regulatory changes happening quickly and often, there is concern that sponsors need more robust systems to keep pace and have a global disclosure strategy in place.

While approved in 2014, Europe's new regulation governing clinical studies is set to take effect in May-leaving interested parties only six more months to prepare for its arrival.

CBI’s conference proves RBM was just the beginning. Now companies are honing in on the risks and mitigations related to clinical trial data reporting.
Globally, 80% of respondents reported that all of their staff had access to a desktop computer for clinical data collection, ranging from a low of 66% in India to a high of 89% in Australia.

EMA and FDA regulatory approvals are just the latest events demonstrating the industry’s growing interest in medication adherence measurement in clinical trials, as well as healthcare in general.

In our latest webcast that aired live October 14, Oracle Health Sciences brought together three presenters from smaller or medium-size organizations in an effort to educate similar-sized companies on the benefits of EDC.

IBM and ICON plc recently announced a collaboration to use the Watson system to enhance oncology clinical trial feasibility, recruitment and start up via Trial Matching.