
The Drug Information Association (DIA) is preparing to celebrate 25 years of training and education provision at its annual EuroMeeting.
Philip Ward is ACT's European editor, phone +44 1244 538583, philipward1@btconnect.com

The Drug Information Association (DIA) is preparing to celebrate 25 years of training and education provision at its annual EuroMeeting.


Criticisms of the pharmaceutical industry's real motives for undertaking post-marketing studies have been made by a special report in the British Medical Journal.



The European Patients' Academy on Therapeutic Innovation faces its first major test during the 2012 Drug Information Association EuroMeeting.

A British Medical Journal survey suggests that misconduct in research is not going away.

The importance of safe use of imaging agents was highlighted at the Chicago Radiological Society of North America conference.

Biobank research has a valuable role to play in R&D, but handling the consent process is rarely straightforward.

UK officials stress importance of evaluating benefits of new therapies before approval.

The EMA is moving its offices to Canary Wharf Estate.

The EFGCP annual conference in January aims to improve information and empower patients.

Guido Rasi, MD, prepares to take on his new role.
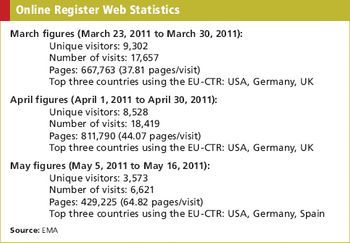
The new online register will help patients access trials information more easily.

Europe's financial problems have resulted in new blackslists of drugs among other challenges.

The recent shutdown of the R&D facility should signify a warning to the British science community.

Patient participation increased drastically in England in 2010.

GCP training will be the main point of discussion at the EFGCP meeting in February.

Some believe that the medical evidence base is distorted by missing clinical trial data.

Pharmacists and patient groups welcome the new European Union directive on pharmacovigilance.

A new report from EFGCP and EUCROF suggests changes to geriatric trials.

The European Medicines Agency has redesigned its Web site to improve transparency.

A major European congress looks set to focus attention on the growing campaign for more participation of the elderly in clinical trials.
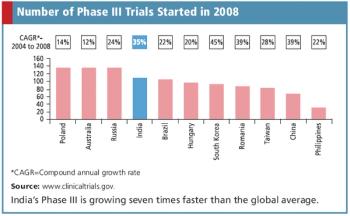
Indian CROs face new guidelines as the country's clinical trial market continues to face infrastructure issues.
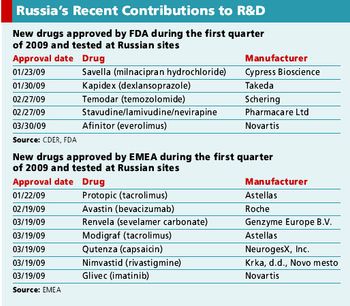
The current state of the clinical trial market in Russia.

The European intiative of involving elderly in clinical trials picks up the pace as challenges are addressed.

Industry professionals address critical commentary of the drug development process.

The Netherlands has become the latest European country to establish a network that is designed to promote pediatric drug development.

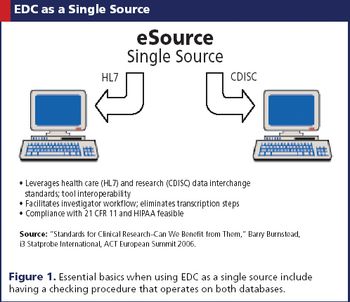
EDC is being embraced by both continents, where its efficiency is welcomed despite a few kinks.