
Declarations made by Beate Wieseler don't align with those of many regulators and HTA bodies in Europe and will prove tough to transform.

Declarations made by Beate Wieseler don't align with those of many regulators and HTA bodies in Europe and will prove tough to transform.

Europe's research ministers are will meet to attempt to reach agreement on European Union research plans for the next seven years, including a cluster devoted to a broad range of health goals that range from general objectives to subjects of more specific interest to the community.

Though members of the industry have made reassuring comments about progress with candidate COVID vaccines, they acknowledge it could be next year, at best, before any real response would be available.

EMA has launched a "communication perception survey" looking for honest views from among the widest audience on how well it is doing.

Amid the COVID-19 pandemic, the EU is allowing trials to start before the requirements of directives dating back as far as 2001 have been met.

Teenagers with cancer could benefit from a proposed initiative to lower the age barrier for participation in trials for new oncology drugs.

Social media uses transparency to highlight alleged gaps in clinical trial transparency and offers a more powerful approach to health campaigners.

Peter O’Donnell explores where the EU stands today in R&D pursuits for new antibacterial therapies.

The regulatory path for virtual studies is nebulous and potentially difficult to navigate-finding a way forward requires a thorough understanding of the terrain and how to apply existing legal frameworks.

Higher screen failure and patient dropout rates are raising the imperative to pilot and implement new study conduct models in trial recruitment and retention.


A shift in emphasis in healthcare strategy could reduce attention and funding to therapy and impose tougher controls over research projects, or possible mean a boost for innovative healthcare. A look at where these trends may go.

The two-year inquiry into possible maladministration at the EMA has intensified.

The consequences UK faces after being pulled out of the EU without any healthcare and research deal may affect all of Europe.

The World Health Organization tries to find a balance with its Road Map for Access on how to best approach research and pricing in Europe.

As elections near, politicians in Europe make promises to prioritize the fight against cancer.

A statement from Europe’s drug industry and Europe’s medical societies calls for a “bold vision” to ensure proper funding and coordination for translational research.

Recent initiatives demonstrate that European health authorities are serious about combatting the proliferation of threats and opportunities from big data.

Survey uncovers deeper learnings of patient perceptions of clinical research and the motivations to participate.

European Union faces a critical view on its pediatric drug development situation, highlighting a lack of treatment, infrastructure, reimbursement, and regulatory agreement.

All of Europe, from MEPs to WHO, aims to develop a comprehensive health policy, with a current priority on nutrition and physical activity.

Blockchain is moving toward definitive uses in clinical trials to enhance clinical supply capabilities, with the potential of enabling data ownership for patients.

A new report on EU's digital health strategy also calls for closer coordination of national HTA arrangements-but both policy initiatives couldn't be more different.

Peter O’Donnell gauges the impact of FDA guidance on igniting the adaptive pathways debate in Europe.



A continued struggle for healthcare and healthcare innovation to be taken seriously by the EU.

Switching from paper records to an electronic drug accountability IRT system can benefit sites during FDA trial site audits.

Assessing the benefits of using blockchain technology as a notary service in the network sharing of clinical data.
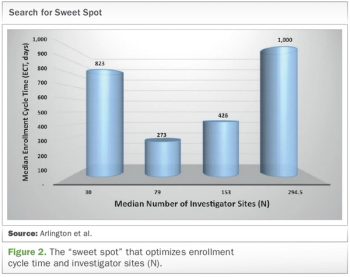
Analyzing data to reveal site performance patterns for better trial planning and execution.