
Columns | View from Brussels


Partnership offers developers full service biosimilar expertise on a global scale

Lisa Henderson interview Gerd Arold or PRA about patient recruitment factors in early phase trials
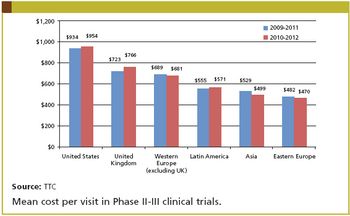


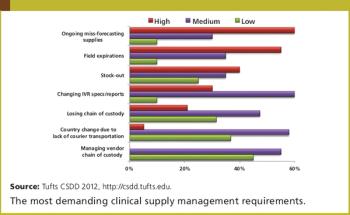
Tufts CSDD

Formulation Considerations for Over-Encapsulation of Clinical Trial Materials in DBcaps® Capsules - Capsugel Whitepaper

The Business of Recruitment

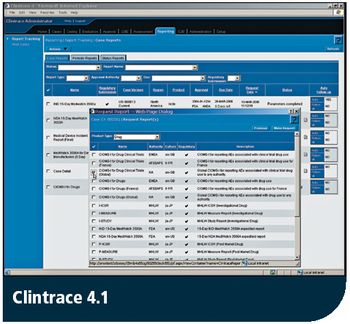
Release takes adverse event reporting to a new level, complies with FDA's new rules

A new report from the European Union grades member states on their orphan drug incentives.

DIA President, Theresa Kane Musser, Executive Director, Development Operations, Rigel Pharmaceuticals, presented the awards

Exhibitor Profiles from the DIA meeting hall

The European pharma industry struggles to regain its former prowess in R&D.

Test images

Andy Richardson, Zenetar Ltd; Case Study: Use of EDC at Boehringer Ingelheim, Stephane Collet, Boeringer Ingelheim, Andrew Cooper, Oracle Corp.;

Presenters included Jean-Pierre Isal, Consultant, UK; Jean-Marc Husson, Prof, Eur Acad of Pharm Med, France; Astra Zeneca. The system has been changed from the Notification System, established in 1988 to a formal Clinical Trial Authorization, 2004-5.

2005 ACT Directory and Buyers Guide

Welcoming attendees to the EuroMeeting were Brigitte Franke-Bray, DIA Director, European branch office, Switzerland; Theresa Kane Musser, DIA President, Rigel Pharmaceuticals, USA; Jean Marimbert, Director General, Afssaps, France; and David Maola, DIA Executive Director. Opening remarks were followed by brief speeches from the two candidates running for DIA President: Ron Fitzmartin, Vice President of Global Technical Services, Daiichi Medical Research, USA, and Dr. Jeffery Sherman, Executive Vice President, NeoPharm, USA.

Orphan drug development may take a back seat to profit and loss concerns.

Pharma's finger of blame once again points outward, never inward.

The pharma industry won't be the only group rewarded for conducting new pediatric research.

Dismay over the level of anti-pharma feeling in the EU is not just "crying wolf."

To present the new scheme as a time-saver rather than a time-waster, the new guidance points out that the data required is a subset of the data required in any case for the request authorization.

Welcome to ACT's Washington DC Exhibitor Profiles Supplement for 2004.

The EU offers up market advantages in exchange for pediatric trials.

In many parts of the globe, intended and actual effects of GCP legislation often differ.

The passing of the EU Directive deadline next month is unlikely to quell the stormy debate over its far-reaching impact.

With increasing authority, the EMEA is indeed living in interesting times.