
Applied Clinical Trials
Russian regulators have reportedly implemented the much awaited changes to the nation's regulatory process for medical devices, and the revised regulations took effect at the start of January.

Applied Clinical Trials
Russian regulators have reportedly implemented the much awaited changes to the nation's regulatory process for medical devices, and the revised regulations took effect at the start of January.

Applied Clinical Trials
To reduce the time from bench to bedside we need to transform competition into cooperation.


Applied Clinical Trials
While there is no single correct way to develop process compliance controls to meet federal clinical trials billing regulations around Medicare, standardization of the entire billing process is key.
Applied Clinical Trials
Options have emerged that make DIY EDC technology more accessible to smaller organizations.

Applied Clinical Trials
The sponsor of a recent Phase II global study, conducted at more than 50 sites, invested in a centralized digital pathology core lab to ensure a homogenous and representative study population.

Applied Clinical Trials
Researchers from sub-Saharan countries are going to have better chances of upgrading their skills.

Applied Clinical Trials
Decision makers can increase collaboration, transparency, efficiency, and effectiveness, while reducing development risks.
Applied Clinical Trials
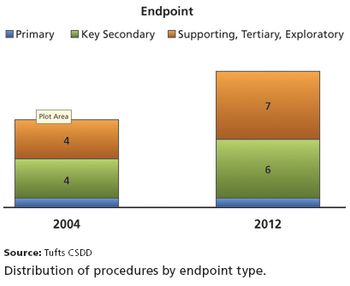
Tufts CSDD

Applied Clinical Trials
The Food and Drug Administration approved nearly 40 new drugs in 2012, beating most recent totals, and is looking for provisions of the FDA Safety & Innovation Act (FDASIA) to further improve its operations and encourage innovation.

Applied Clinical Trials
Options have emerged that make DIY EDC technology more accessible to smaller organizations.
Applied Clinical Trials
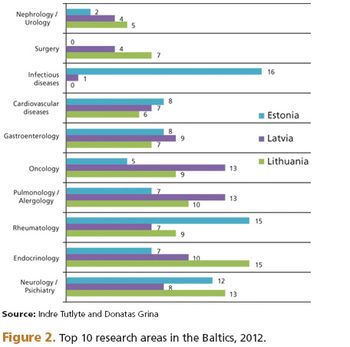
The region has an appropriate population size, solid infrastructure, experienced nvestigators, and short timelines.

Applied Clinical Trials
CRO/SPONSOR : Trials in the Baltics eCLINICAL : DIY EDC Technology, Evaluation and Selection Also in this issue : Transforming Competition into Cooperation, Fellowships in Africa, Trial Planning and Execution