
Applied Clinical Trials
Digital media is becoming a crucial vehicle for finding “Patient X” for rare disease drug trials. Three areas in particular where digital media is helping with the detective work are explored.

Applied Clinical Trials
Digital media is becoming a crucial vehicle for finding “Patient X” for rare disease drug trials. Three areas in particular where digital media is helping with the detective work are explored.

Applied Clinical Trials
Rare disease interests in Europe add more voices to push for greater backing of research incentives, infrastructure.

Applied Clinical Trials
Peter O’Donnell weighs the varying views in Europe on the risks of going the adaptive pathways route for drug approval.

Applied Clinical Trials
The ability to diagnose cancer at earlier stages of the disease is constrained by the specificity and mechanics of traditional diagnostic methods. The growing use of liquid biopsy technology may help overcome such barriers and potentially revolutionize cancer research, patient treatment, and survivor care.

Applied Clinical Trials
Safeguarding trials from CRO turnover. How to ensure commitment of qualified resources through thick and thin.

Applied Clinical Trials
Lisa Henderson writes on patient participation and recruitment in clinical trials.

Applied Clinical Trials
The importance of examining this generation’s influence on the clinical trial value chain.
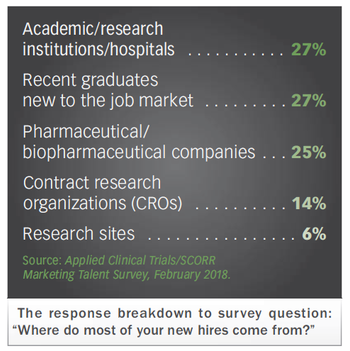
Applied Clinical Trials
Exploring the latest hiring and retention practices in the life sciences, where roles are changing fast. Impact trends from new talent survey are revealed.

Applied Clinical Trials
Amid industry feedback that the growing volume and diversity of eClinical data collected for studies is taxing cycle times, two studies highlight the need to optimize protocol design and executional complexity to overcome these data management burdens.

Applied Clinical Trials
Jill Wechsler details the two chief reasons why clinical trial quality and efficiency has improved in recent years.
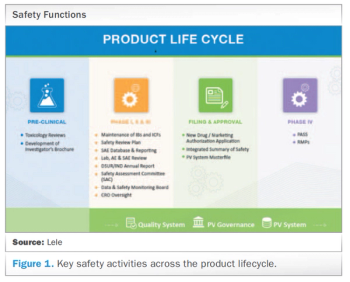
Applied Clinical Trials
Meeting today’s complex regulatory demands when it comes to drug safety and pharmacovigilance can be especially challenging for small and medium-sized organizations. This report presents the benefits for these companies in outsourcing such activities to functional service providers (FSPs) during clinical trials and post-approval.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials March 2018 issue in an interactive PDF format.