
Applied Clinical Trials
The "Cures" bill and PDUFA IV are the latest measures pushing for more systematic inclusion of patient views into drug development.

Applied Clinical Trials
The "Cures" bill and PDUFA IV are the latest measures pushing for more systematic inclusion of patient views into drug development.

Applied Clinical Trials

Applied Clinical Trials
While still early days, key metrics can be collected in three broad areas

Applied Clinical Trials
Officials tackle such areas as early diagnosis and treatment, patient access, and personalized medicine

Applied Clinical Trials
Combining PRO data with clinical feasibility data can make a difference in diabetes care.
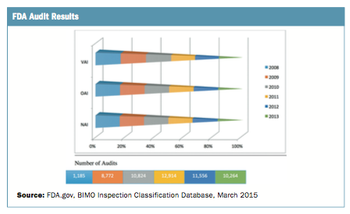
Applied Clinical Trials
The number of site audits performed has grown significantly.

Applied Clinical Trials
The four essential steps to ensuring optimal clinical deliverable specifications and process controls.

Applied Clinical Trials
How to satisfy regulatory concerns about EDC data integrity and site controls over its source records.

Applied Clinical Trials
Whether we are referring to CSRs or to IPD, the personal information of trial participants needs to be de-identified prior to release. This article will describe the available methods for de-identifying clinical trial data, and the relative strengths and weaknesses of each.

Applied Clinical Trials
CRO/Sponsor: Logical Sourcing: Key Steps Clinical Technology: eSource Record-Keeping Data Sharing: De-identifying Trial Data Also in this issue: R&D Focal Points in Europe Patient Centricity: The Clinical Investment PRO Plusses for Diabetes Research