
Applied Clinical Trials
Comparing mean vs. median to uncover the full data picture of site-level performance.

Applied Clinical Trials
Comparing mean vs. median to uncover the full data picture of site-level performance.

Applied Clinical Trials
With a new administration in Washington, sponsors and regulators are weighing several initiatives that promise to reshape clinical research policies.

Applied Clinical Trials
The Council adopts revision encouraging sponsors to implement improved oversight and management of clinical trials.
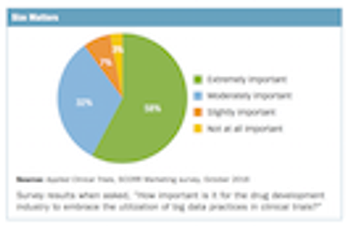
Applied Clinical Trials
Survey collects thoughts from industry professionals on the current and future impact of big data in clinical trials.

Applied Clinical Trials
With bidding starting to intensify among member states, several factors will determine the EMA’s next home.

Applied Clinical Trials
For those still waiting, the time is now to get compliant on FDA's new data submission requirement.

Applied Clinical Trials
New study finds that the long-strained relationship between sites and institutional review boards may finally be improving.
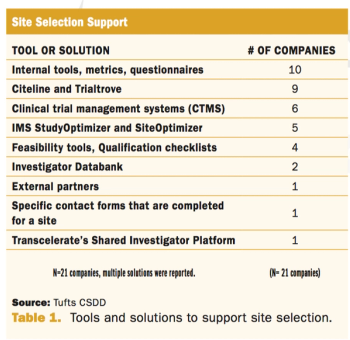
Applied Clinical Trials
Assessing practices and inefficiencies with site selection, study start-up, and site activation.

Applied Clinical Trials
How one large academic trial adopted a coordinating center model-helping drive early-model RBM gains

Applied Clinical Trials
Q&A looks at the growing use of unstructured data in drug development and the evolving role of the clinical data manager amid the advent of real-world data as a research aid.

Applied Clinical Trials
In its first published update since 2011, the CDISC Glossary Project Team has updated this resource, which includes hundreds of definitions for key terminology related to clinical research.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials December 2016/January 2017 issue in an interactive PDF format.
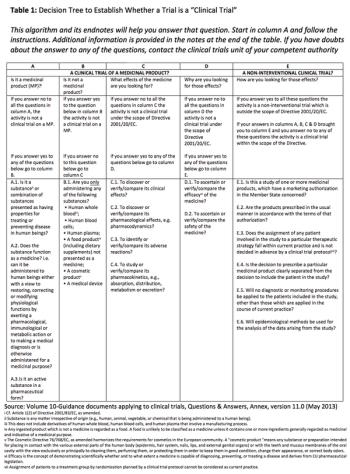
Applied Clinical Trials
Examining interventional vs. non-interventional clinical study classification in the EU.