
BBK Worldwide and CROee today announced the creation of a jointly-owned US-based company, BBK + CROee.

BBK Worldwide and CROee today announced the creation of a jointly-owned US-based company, BBK + CROee.

Bi-directional Integration with Oracle Health Sciences InForm Enables Real-time Clinical Trial Data Visibility and Reduces Data Management Costs

Bi-directional Integration with Oracle Health Sciences InForm Enables Real-time Clinical Trial Data Visibility and Reduces Data Management Costs

Aspire IRB and Midlands IRB become part of the WCG family, further expanding its geographic and program reach

Measuring Home Spirometry combined with Administration of ACQ and/or EXACT-PRO Increases Data Reliability while Reducing Trial Costs

Wireless Solution Delivers Cost Savings and Real-time Access to Key Patient Data

Latest RTSM technology features standardized study components for faster implementation and greater design flexibility
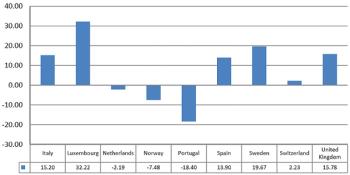
In some countries, the participation in clinical trial, is still the only possibility to be treated.

But the re-brand of PRA Health Sciences from PRA International strikes a different tone than most.

ArisGlobal has opened up new opportunities for clinical trial sponsors to use agDisclosure to meet trial registration and disclosure requirements at no cost.

PatientsLikeMe has unveiled a suite of services for pharmaceutical companies that allows them to collaborate with patients on the design of clinical trials and other research.

DIARYpro Available on Patients' Smartphones - Delivering Reliability while Reducing Trial Costs

MasteScope 2.0 Simplifies Complex Clinical Trials, Enabling Investigative Sites to Focus on Patients and Data Quality

The Future of Clinical Development Today

Research shows advanced eTMF technologies improve inspection readiness and compliance while reducing costs

INC Research invites DIA attendees to join them at the DIA Innovation Theater today at 1-2 pm.

Novotec has signed an MOU with the Korea Drug Development Fund to promote the development of the Korean biotechnology sector in the Asia Pacific region.

MCC released an in-depth report on risk-based monitoring practices.

Mobile Apps to Streamline Valuable Resource and Information Sharing, Better Connect Advocacy Groups, Pharmaceutical Sponsors and Patients

SCRS Global Impact Partner program announced it has gained four new members since April 1.

Forte Research Systems announces its newest offering, the Allegro Clinical Trial Search.

Forte Research Systems, Inc. is happy to announce its newest offering, the Allegro Clinical Trial Search.

SCRS Welcomes INC Research, inVentiv Health, Novartis and Sanofi

MCC continues to lead the industry in the development, adoption and utilization of standardized metrics and tools to drive performance improvement
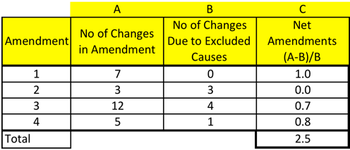
This month, let's look at a quality metric that's critical to on-time, on-budget performance: protocol amendments.

Industry news focusing on the people and organizations who work in the clinical trials profession.

The Regulatory Affairs Professionals Society and the National University of Singapore have announced the official launch of their joint Graduate Certificate in Medical Devices Regulatory Affairs program.

RAPS and the National University of Singapore have announced the official launch of their joint Graduate Certificate in Medical Devices Regulatory Affairs program.

EMA Management Board to formally adopt policy in coming weeks

Thomson Reuters Cortellis Competitive Intelligence