News


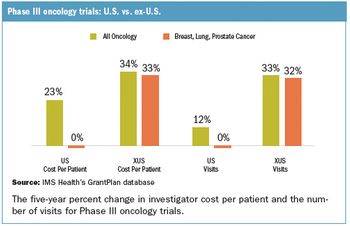
Longer-term strategic issues are making already high-investment, high-risk decisions to place studies overseas even more complex.

Unique feedback from patient survey could help inform future clinical supply design and implementation.
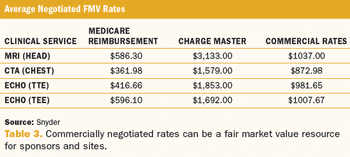
Breaking through the confusion around fair market value when applying the concept to study budgets.

CRO/Sponsor: Clinical Supply Survey, Strategies Sites: Fair Market Value Debate Clinical Technology: Optimizing Supply Chain Data Trial Design: Multi-site Pre-implementation Also in this issue: European Innovation Initiative, Part II Medicine Kits: The Patient Perspective Global Trials Need Strategic Touch

Synexus has opened a research center in Bochum.

Synexus has opened its 23rd Dedicated Research Centre in Bochum

As Europe departs for its annual holiday the question remains unresolved as to what to expect from the new European Parliament in terms of the principal interest of readers of Applied Clinical Trials.

In mid-July, the SAS Press Program released a new book titled Risk-Based Monitoring and Fraud Detection in Clinical Trials Using JMP and SAS.

Hangzhou Tigermed Consulting Co., Ltd. and Frontage Laboratories, Inc. today announced completion of the previously announced investment agreement.

Hangzhou Tigermed Consulting and Frontage Laboratories announced completion of the previously announced investment agreement

The patent cliff that rocked big pharma is now starting to reverberate in companies developing generic alternatives.
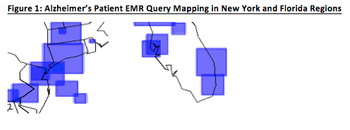
The topic of subject enrollment has been evaluated from many different perspectives.

After months of speculation about prospects for biosimilar development in the US, Novartis announced July 24 that FDA has accepted Sandoz' biologics license application ...

It's a given that events will happen during the course of the trial that will require not just new documents, but new sets of documents.

ESMO concerned that the proposed EU General Data Protection Regulation could make cancer research impossible and add a significant burden to cancer patients

ESMO is concerned that the proposed European Union General Data Protection Regulation could make cancer research impossible and add a significant burden to both doctors and cancer patients.

Expanding bio-risk management support for federal, state and institutional laboratories

Astra Nova Training and ACRES announced a strategic alliance collaboration

This article will discuss FDA's post-marketing surveillance programs, and how biopharmaceutical sponsors can work with the FDA to identify unreported Adverse Events.

Cognizant announced that it has been selected by TransCelerate BioPharma Inc. to develop a subscription-based platform

The Shared Investigator Platform, Built as an Industry Utility, Will Help Accelerate the Development of New Medicines

Astra Nova Training and ACRES announced a Strategic Alliance collaboration.

More attention is being paid to blood pressure effects in new chemical entities under development for non-hypertension indications.

Adds largest independent and only AAHRPP-accredited Canadian IRB/REB to the Chesapeake IRB organization

Combined Assets Expand Expertise and Reach

CTI Clinical Trial and Consulting Services announces that it has opened an office in S?o Paulo, Brazil

Chesapeake IRB has acquired Goodwyn IRB and IRB Services

CTI Clinical Trial and Consulting Services has opened an office in Sao Paulo, Brazil.
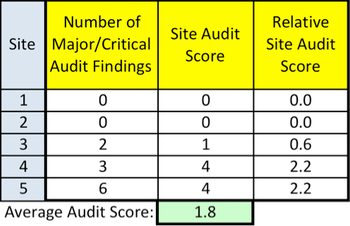
MCC has just published an executive summary of its Risk Based Monitoring Usage Survey.