It is estimated that by the end of 2014, pharmaceutical companies will lose approximately $78 billion USD as a result of medications going off patent.

It is estimated that by the end of 2014, pharmaceutical companies will lose approximately $78 billion USD as a result of medications going off patent.

CluePoints selected by the Nagoya University School of Medicine to assess data quality and integrity

EMC announced Documentum Submission, Store and View, for simplified storage, search, and retrieval of submission documentation and all associated correspondence...
A growing number of providers are adapting 21st century web-based and mobile tools to connect the corners, boost trial participation, and speed trial completion.

Cenduit announces the delivery of its newly-designed IRT system interface

PAREXEL announced that clinical technology companies CRF Health and Clinical Ink have joined its Perceptive Partner Program

Life sciences service providers leverage PAREXEL's eClinical solutions to simplify drug development for customers through technology partnership initiative

Why do we audit our suppliers and what do we hope to achieve when we do?

Cenduit announced its newly-designed IRT system interface

Blue Chip Patient Recruitment and J Trotter Research & Consulting have merged

WIRB-Copernicus Group announced that New England Independent Review Board has joined its group of companies.

It's no secret that there is increased clinical focus now on the prevention and early detection of Alzheimer's.

Citeline reported that clinical trial transparency is in a much healthier state than shown in previous studies.

Medidata and ICON announced a joint initiative

PRA Health Sciences announced that it has filed a registration statement with the US Securities and Exchange Commission

Joint ePRO Initiative Delivers Ready-to-Use, Patient-Centric Solutions to Speed Trial Times and Enhance Patient Engagement


Leading healthcare firms Blue Chip Patient Recruitment and J Trotter Research & Consulting combine to form Continuum Clinical.

New addition expands WCG's New England presence and increases its expertise in all phases of clinical research
It should suffice to say that obtaining informed consent is required ethically and legally in almost all forms of human research in almost all countries.

Efforts by drug companies to streamline and improve the execution of clinical study designs-to counter mounting costs and shorten development times associated with bringing new drugs to market-are yielding positive benefits.

Efforts by drug companies to streamline and improve the execution of clinical study designs-to counter mounting costs and shorten development times associated with bringing new drugs to market-are yielding positive benefits.

Eye for Pharma hosted its first Patient Centered Clinical Trials symposium in Boston.

The European Forum for GCP and MedTech Europe have established a Medical Technology Working Party.

Select Clinical Trials announced the launch of its website designed to help identify clinical trials for common illnesses and medical conditions

Select Clinical Trials, a public service for all individuals interested in medical research, launched a new website that catalogues leading clinical trials conducted by doctors and healthcare professionals that the public can participate in.

New multi-stakeholder collaboration will drive dialogue on specificities of clinical standards for medical technology

Wingspan Technology released eTMF 2.1 on August 29.
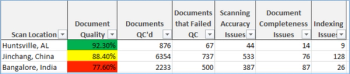
Even though sponsors and CROs are working to create more and more documents as electronic originals, scanned paper still comprises a large portion of any given eTMF.