
A Medical Technology Working Party is established to promote collaboration and dialogue on the specific aspects of clinical standards.

A Medical Technology Working Party is established to promote collaboration and dialogue on the specific aspects of clinical standards.

Clinical Conducter CTMS and OpenClinica are pleased to announce the formation of a strategic partnership
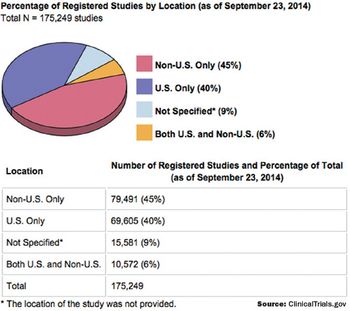
The distribution of locations for all trials registered on ClinicalTrials.gov.


ACRP announced changes to its investigator certification program

ACT 2015 E-Newsletter Schedule

ACRP announced changes to its investigator certification program

Clinical Conductor CTMS and OpenClinica will deliver a seamless EDC-CTMS integration under a new strategic partnership.

ACT 2015 Rates

LabConnect, LLC has released its BioVisualization software platform

Trial Design: Identify Blood Pressure Effects Early Demonstrating Biosimilarity CRO/Sponsor: Data Monitoring Committee Users Guide Also in this issue: EU Reshuffling Impacts Pharma "Open Integration" Promise Elusive Assessing Drug-Impaired Driving

This e-Book features information from a recent conference of global thought-leaders in clinical data disclosure and transparency, which address current practices, potential challenges and solutions for pharmaceutical companies complying with the regulatory, ethical and legal issues around clinical trial data sharing.

IACRN and ACRES announced the signing of a strategic alliance agreement

IACRN and ACRES have created a strategic alliance agreement.

Chiltern has acquired Pacific Clinical Research, a pan-Asian CRO based in Singapore.
ClinicalTrials.gov

Chiltern has acquired Pacific Clinical Research

Two main themes arose from the ePatient Connections Summit: online patient engagement and patient centricity.

ACRP and CenterWatch released a collaborative benchmarking report on clinical research site performance.

With numerous strategic partnerships underway, X-Chem emerges as a leading biotechnology company focused on drug discovery

Radiant Research/CRA has completed enrollment for the Dynavax Technologies Phase III clinical trial

ACRP and CenterWatch released a collaborative benchmarking report on clinical research site performance

With numerous strategic partnerships underway, X-Chem emerges as a leading biotechnology company focused on drug discovery

Radiant Research/Clinical Research Advantage has completed enrollment for the Dynavax Technologies Corporation phase III clinical trial

Effective leadership is needed in a trial leader, but what exactly does that entail?
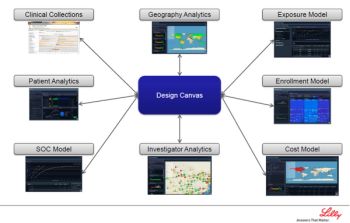
Thomas Krohn, Director of Clinical Open Innovation at Eli Lilly, showcased a breakthrough study design platform and process that study teams are currently utilizing.
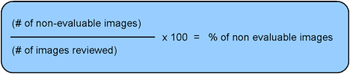
Medical imaging is an important source of subject eligibility, drug efficacy, and safety data for many clinical trials.

Key differences and gaps in requirements for testing and documenting product similarity have emerged among the European Union, the US and other regions.

New addition expands WCG's eClinical offerings for improving clinical trial start-up and management

PHT Corporation announced its LogPad System has been selected by Agile Therapeutics for use