Cohen addresses the three-pronged parts of drug development process or clinical trials

ClinicalTrials.gov

Provectus Biopharmaceuticals Collaborates with ERT's COA Experts to Understand Patient Perspective during Melanoma Drug Development

Rep. Renee Ellmers 21st Century Cures Round Table - Vaccine Focus

Strategic relationships between drug developers and contract research organizations are generating innovative approaches to clinical trial design and execution

The Association of Clinical Research Professionals (ACRP) has responded to the editors of The Toronto Star in regard to its recent news and editorial coverage of Health Canada and the pharmaceutical industry in Canada.

The Association of Clinical Research Professionals (ACRP), one of the largest representative organizations for clinical research professionals in the world, today submitted the following letter to the editors of The Toronto Star in response to recent news and editorial coverage of Health Canada and the pharmaceutical industry in Canada.

Novotech, Australia?s largest clinical CRO with offices throughout Asia, announced today the expansion of services to China with a Novotech office in Shanghai, a wholly owned entity with local staff. Shanghai is the ninth Novotech office in Asia.

INC Research, a global Phase I to IV contract research organization, has filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission (SEC) related to a proposed initial public offering of its common stock.

INC Research Holdings, Inc. (INC Research), a leading, global Phase I to IV contract research organization, today announced it has filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission (SEC) related to a proposed initial public offering of its common stock. The number of shares to be offered by INC Research

The Alliance for Clinical Research Excellence and Safety (ACRES) has opened its first national affiliate, ACRES Japan, in Tokyo. Fumimaro Takaku, M.D., president of the Japanese Association of Medical Sciences, will act as president of the board of directors, and ACRES Japan will provide a model for the organization?s planned network of national affiliates.

Novotech, Australia?s largest clinical CRO with offices throughout Asia, announced today the expansion of services to China with a Novotech office in Shanghai, a wholly owned entity with local staff. Shanghai is the ninth Novotech office in Asia.

The Alliance for Clinical Research Excellence and Safety (ACRES), a Massachusetts-based non-profit operating in the public interest, has taken the first step in its plan to create a globe-spanning framework of regional and national affiliates with the opening of its first national affiliate, ACRES Japan, in Tokyo last week.

Training providers meeting the GCP criteria set out in the guidelines are added to a list of mutually recognized providers. The Clinical Research Network has recently been added to this list. Now, when a study is placed at a site in the NHS, if the Principal Investigator and the research team have completed GCP training provided by the Clinical Research Network, they will not be required to undertake further company-specific GCP training. This will help to streamline study set-up and allow research teams to focus on other important set-up activities such as pre-screening patients.

The National Institute for Health Research (NIHR) Clinical Research Network?s Good Clinical Practice (GCP) training has been added to the list of programmes mutually recognised by TransCelerate member companies. The principles of ICH-GCP is an international standard for the conduct of clinical research. Undertaking clinical research in compliance with these principles provides assurance that the rights, safety and well-being of trial subjects are protected, and that the results of the clinical trials are credible and accurate.

PAREXEL International Corporation (Nasdaq: PRXL)(?PAREXEL? or the ?Company?), a global clinical research organization, announced today that the Company has acquired all of the outstanding equity securities of privately-owned ClinIntel, a provider of clinical Randomization and Trial Supply Management (RTSM) services, based in the United Kingdom.

Bio-pharmaceutical companies are continually tasked with gathering more safety information and responding to requirements more quickly. Recent examples of significant changes in reporting requirements include 2010/84/EU and Regulation Number 1235/2010 on pharmacovigilance, and the 2012 European Medicines Agency (EMA) new guidelines on good pharmacovigilance practices.

Parexel has acquired privately-owned ClinIntel, a provider of clinical Randomization and Trial Supply Management (RTSM) services, based in the United Kingdom. ClinIntel?s offerings will be combined into the ClinPhone? RTSM suite

Bio-pharmaceutical companies are continually tasked with gathering more safety information and responding to requirements more quickly. Recent examples of significant changes in reporting requirements include 2010/84/EU and Regulation Number 1235/2010 on pharmacovigilance, and the 2012 European Medicines Agency (EMA) new guidelines on good pharmacovigilance practices.

The European Medicines Agency (EMA) has decided to publish the clinical reports that underpin the decision-making on medicines. Following extensive consultations held by the Agency with patients, healthcare professionals, academia, industry and other European entities over the past 18 months, the EMA Management Board unanimously adopted the new policy at its meeting on 2 October 2014. The policy will enter into force on 1 January 2015. It will apply to clinical reports contained in all applications for centralized marketing authorisations submitted after that date. The reports will be released as soon as a decision on the application has been taken.

Korea National Enterprise for Clinical Trials (KoNECT) and DIA (Drug Information Association) have signed a Memorandum of Understanding to establish a cooperative relationship and explore collaboration in development of training, education and knowledge exchange opportunities.

Korea National Enterprise for Clinical Trials (KoNECT) and DIA (Drug Information Association) have signed a Memorandum of Understanding to establish a cooperative relationship and explore collaboration in development of training, education and knowledge exchange opportunities.

The EMA press release issued states: "According to the policy?s terms of use, the public can either browse or search the data on screen, or download, print and save the information. The reports cannot be used for commercial purposes. In general, the clinical reports do not contain commercially confidential information.

Leading international experts are organizing a new high-level two-day meeting about the interdisciplinary challenges faced in pediatric drug development. The plan is for the meeting to become an annual event. Called ?Development of Medicines for Paediatric and Rare Diseases,? the conference will take place in Basel, Switzerland, on?February 3-4, 2015

ACT 2015 Editorial Calendar

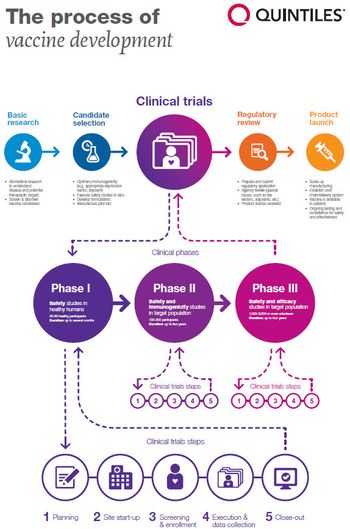
The rush is on to develop new therapies and vaccines to combat the lethal outbreak.

ACT 2015 Print Rates

LabConnect, LLC announces the release of its BioVisualization software platform.

The federal Open Payments program went live Sept. 30.

ACT 2015 Editorial Calendar