
Global packaging company has received a grant from The Bill & Melinda Gates Foundation to improve adherence and accuracy during PrEP trials

Global packaging company has received a grant from The Bill & Melinda Gates Foundation to improve adherence and accuracy during PrEP trials

Performer platform enables users to benchmark clinical trial performance against industry standards, assess key drivers of performance, and provide predictive analytics

CRO Analytics announced that it is seeking sponsor and CRO partners to enroll in a limited Pilot to Production program

Global packaging company has received a grant from The Bill & Melinda Gates Foundation to improve adherence and accuracy during PrEP trials
There is a lot of discussion and awareness on the topic of patient centricity, as many experts are starting to discuss how the concept can improve clinical trials.

The drug industry has seen an increasing movement to leverage mobile technology to engage with patients and collect their data during clinical trials.

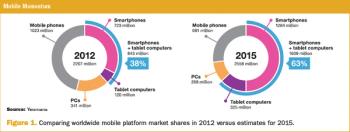
It is tempting to imagine the use of the patient's own mobile computing platform for collection of patient-reported outcomes (PROs).

The promise is that customized delivery of injectible drugs and biologics will reduce toxicity, enhance individual response, facilitate the delivery of multiple drugs, minimize waste, and encourage patient adherence to prescribed treatment.

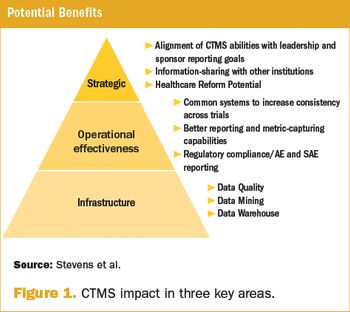
As the importance of translational research continues to grow, research institutions are exploring other options to remain competitive players in the academic clinical research arena.


The Future of ePRO Collection Mobile Customization Strategies Academic Research and CTMS Also in this issue: New Combination Drug Challenges Clinical Research Perceptions in Europe Key Benefits of Investigator Portals

Applied Clinical Trials offers this e-Book as the definitive resource for the latest perspectives on risk-based monitoring. As acceptance for risk-based monitoring grows, this e-Book examines next steps for sponsors and CROs. This e-Book offers case studies, practical tips, and applications to enhance the effectiveness of a risk-based monitoring strategy.

Applied Clinical Trials 2015 E-media Kit

Industry news and announcements for people in the clinical trials industry.

AiCure has announced the start of a major clinical trial to monitor and intervene with patients receiving medication as maintenance therapy for opioid addiction

Sponsors Requesting 24/7 Medical Support & Emergency Unblinding can Save Time and Money

The two-year-old initiative to accelerate the development and approval of highly effective drugs and biologics has enabled a number of important new medicines to reach patients sooner, according to Janet Woodcock.

AiCure has announced the start of a major clinical trial to monitor and intervene with patients receiving medication as maintenance therapy for opioid addiction.

Almac and ESMS have combined their expert knowledge to offer clients an exclusive solution surrounding emergency unblinding procedures.

Experts to submit updated declarations of interests by end January 2015

EMA has published its revised policy on handling declarations of interests for scientific-committee members and experts.

Veeva Systems introduces innovations to its Veeva Vault product for regulated content management for life sciences.

The integration of Chesapeake IRB and IRB Services is now complete.

Veeva Vault balances global harmonization with local autonomy for regions, departments, therapeutic areas

Cloud-based platform integrates operations in the United States and Canada.

US researchers and regulators continue to support the use of randomized clinical trials to test potential treatments and vaccines to combat the Ebola virus...

The most advanced IRT user interface available.

YPrime, LLC released its latest addition to the YPrime clinical research software suite.