
CROMSOURCE announced that it is delivering several modules of a training course on clinical research best practice organized by the SIFC.

CROMSOURCE announced that it is delivering several modules of a training course on clinical research best practice organized by the SIFC.

The EuroMeeting and Clinical Forum will take place in Paris as planned, in spite of January’s terrorist attacks in the French capital.
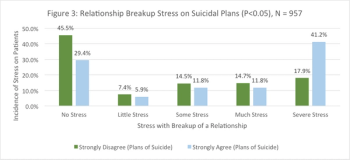
This article will analyze factors that affect suicidal ideation from a clinical psychiatric pilot study.
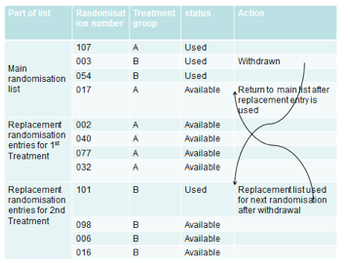
The drive to accelerate drug development has highlighted the benefit in using RTSM systems within early phase cohort trials.

Investigators and sponsors of clinical trials will have to make more detailed data available following study completion, according to a new report from an Institute of Medicine expert panel.
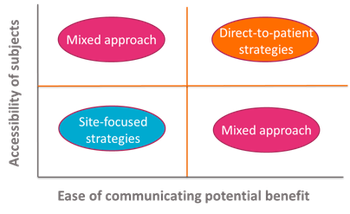
It is well documented that lengthy start-up at individual trial sites related to contracting and committee reviews contributes significantly to delays.

Systemwide Enhancements Deliver Exceptional Configurability, Performance and Efficiency

MedNet Solutions has released the latest version of its iMedNet™ eClinical, its cloud-based technology platform.

Almac announced the results of a survey conducted by researchers at the Tufts Center for the Study of Drug Development.

Almac announced the results of a survey conducted by researchers at the Tufts Center for the Study of Drug Development.
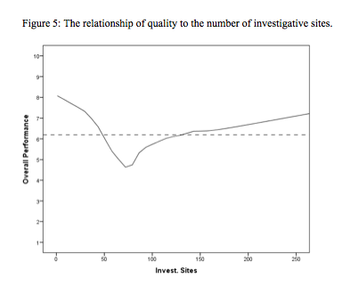
This article is the first in a series of articles that will review the development and findings of the first scientific measurement of quality in clinical trials.

Novella Clinical and the Cardiovascular Research Foundation announced a preferred provider collaboration.


Professor Sirpa Leppä pointed to the increasing challenge of inducing trial sponsors to select Finland in recent article.

Over half of top 30 pharma and world’s largest CROs now using Activate worldwide

goBalto released its latest version of goBalto Activate.

Oracle and Proteus have integrated Proteus Digital Health Feedback System with Oracle Health Sciences InForm.

Oracle Health Sciences InForm electronic data capture platform utilizes Proteus solution, enabling greater accuracy in measuring medication adherence during clinical trials.

This guidance document will offer information about RbM strategies.



Number of medicines with new active substances continues to increase.

Director General of EFPIA argued passionately in favour of the homogenization of European HTA bodies and regulation, during a recent interview

The Director General of EFPIA has spoken out strongly in favor of the homogenization of European health technology assessment bodies and regulation.

Novartis selects Qualcomm Life and its 2net technology to digitize its clinical trials

In 2014, the European Medicines Agency (EMA) recommended the highest number of orphan designated medicines for marketing authorization in a year.

Qualcomm announced that its subsidiary, Qualcomm Life, has been selected by Novartis as a global digital health collaborator for its Trials of The Future program.
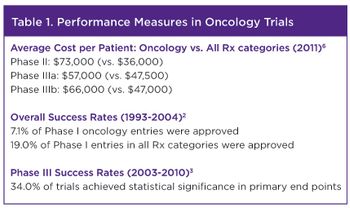
Today’s rich oncology pipeline-accounting for nearly 25% of agents in clinical development-promises much needed advances in cancer therapy.

Radiant Sage announced the release of its Clinical Trial Management System RadClinica™ version 3.0

Clinical Trial Management Solution Now Offers a Robust and Intuitive System with Real-time Information to Organize a Trial