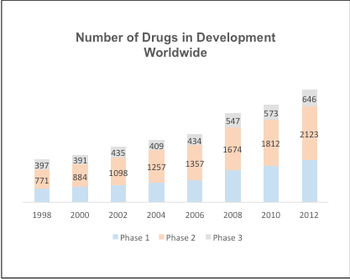
How the use of eSource, eTMF, and other cloud-based solutions are changing clinical trials

How the use of eSource, eTMF, and other cloud-based solutions are changing clinical trials



Through a partnership with Lilly USA, third- and fourth-year medical students from IU School of Medicine are learning about drug development and how the many physicians working at Lilly play different and important roles in bringing new and innovative medicines to patients.


System provides nimble, efficient review of multi-centre clinical trials, while maintaining highest ethical standards


Verona, Italy-based technology company Techorizon, announced the launch of FeRMI, an electronic application to advance the conduct of clinical trial feasibility studies.



Intrinsic Imaging is the only imaging core lab that is ISO 13485 certified specifically to provide quality services for medical device clinical trials.

CRF Health, the leading global provider of eCOA solutions for the life sciences industry, today announced a complete TrialMax® eCOA solution for diabetes clinical trials.

Building on the most widely leveraged standardized reference in TMF management today, with version 2.0 used by more than a hundred life science sponsors, CROs and technology vendors, the next major release of the TMF Reference Model will incorporate feedback from its extensive industry

Many years before the FDA offered its eSource Guidance in September 2013, Sirion Therapeutics launched its first two pivotal Phase III studies using entirely electronic source data collection.

A new provider of clinical studies and strategic consulting, Orange OTC Research, aims to shake up the market for over-the-counter (OTC) medicines and related products.


Certara®, a global biosimulation technology-enabled drug development and drug safety consulting company, announced that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) is equipping its pharmacometrics team with Certara’s Phoenix® biosimulation solutions and its Simcyp™ Population-based Simulator.


Catalent Pharma Solutions, the leading global provider of advanced delivery technologies and development solutions for drugs, biologics and consumer health products, has today announced several expanded capabilities within its European Clinical Supply Services network.

The Randomized Clinical Trial (RCT) is considered the gold standard of research and adds credibility to the efficacy or effectiveness of an investigational new drug. However, with a growing need for post-marketing safety databases, and increased need to collect qualitative information from patients in the pre- and post-market of a drug the evidence gathering from clinical trials onward is expanding. This eBook examines the growing trends of evidence gathering and how that impacts the clinical trials industry.


ERT, a leading global solution provider for high- quality patient safety and efficacy endpoint data collection, and PHT Corporation (PHT), the eClinical innovator leading the adoption of patient-driven mobile apps for improved clinical research, announced today that they have signed a definitive agreement under which ERT will acquire PHT.

It seems only yesterday that the future for European pharma was personalized medicine. The European Union first gave the term official status in a formal paper in 2008, entitled a Renewed Vision for the Pharmaceutical Sector, in which the Commission included a section on ‘Towards more personalized medicines’.
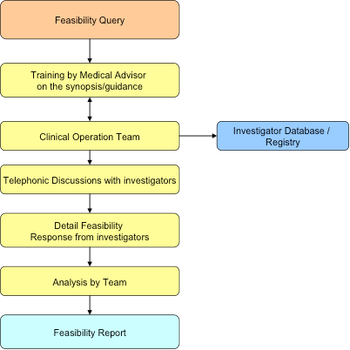
The current regulatory environment of a particular country plays an important role in the geographic selection. For India, it is very relevant because of problems in clinical trials approval and conduct issues during last few years.

A critical part of clinical trial conduct is providing medical guidance by the responsible medical monitor to investigative sites and various operations team members, including, but not limited to, clinical research associates and clinical study managers.


The Alliance for Clinical Research Excellence and Safety (ACRES) announced the launch of ACRES BlueCloud™

TransCelerate BioPharma Inc. has selected DrugDev to develop and host its Investigator Registry
