
ArisGlobal, a leading provider of solutions to the life sciences industry, has today announced the availability of agFileMaster; a Cloud-based electronic trial master file.

ArisGlobal, a leading provider of solutions to the life sciences industry, has today announced the availability of agFileMaster; a Cloud-based electronic trial master file.

The team behind Clinical Conductor CTMS recently debuted new functionality that allows healthcare organizations to better manage adaptive and dynamic clinical research that is becoming more relevant in the industry every year.



Three new academic programs have been added to the PAREXEL Academy which span the globe and are designed to prepare students for careers in the biopharmaceutical industry.
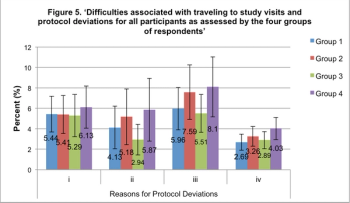
Survey shows what keeps Ukrainians away from clinical trials

The use and number of cognitive-enhancing drugs is likely to grow substantially in the coming years, and researchers must prioritize the potential advantages and dangers of their use in healthy subjects, according to U.K. neuroscientists writing in The Lancet Psychiatry journal.

In a crucial part of the European Patients’ Academy (EUPATI) strategy, 47 patients and patient representatives from across Europe are meeting this week in Barcelona, Spain, to continue their 13-month online training course to become expert patients in the medicines development process.


Denis Lacombe, M.D., has been appointed Director General of the Brussels-based European Organisation for Research and Treatment of Cancer (EORTC).

TransCelerate BioPharma has established two new global initiatives in the clinical trials area. Both initiatives - Placebo / Standard of Care Data Sharing and Electronic Labels for Clinical Trials (e-Labels), are made possible through the collaboration of participating Member Companies.

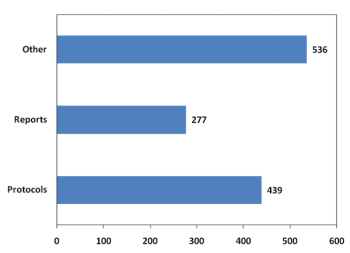
In a move designed to both tout the importance of precision and to improve the sponsor experience in conducting cardiac safety assessment, iCardiac Technologies announced a full risk-sharing program for Thorough QT studies.

Sponsors Are Guaranteed to Receive Precise, Conclusive Thorough QT Results or Pay Nothing

The use and number of cognitive-enhancing drugs is likely to grow substantially in the coming years, and researchers must prioritize the potential advantages and dangers of their use in healthy subjects, according to U.K. neuroscientists writing in The Lancet Psychiatry journal.



TrialScope, a clinical trial transparency and compliance solutions provider, announced the availability of Convert, a free online clinical trial data conversion service.

Throughout this trial, YPrime will be supporting world class selected sites to conduct the study with data driven metrics pulled from these tools. YPrime is dedicated to applying its leading edge eClinical technology to help forward unmet medical needs in the Biotech and Pharmaceutical industry.

Triumph Research Intelligence (TRI), based in London, UK and Raleigh, NC announces its Risk-Based Monitoring platform, Operational Platform for Review, Reporting and Analysis OPRA, for a combination of statistical calculations, data visualizations and operational activity management.

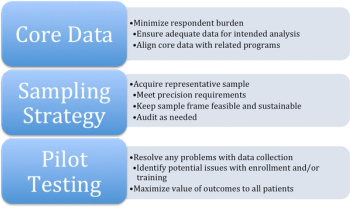
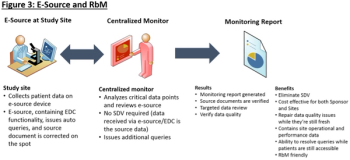
The emergence of risk-based monitoring (RbM) is creating a revolution in the way biopharmaceutical sponsors and CROs manage clinical trial quality.

Certara®, a global biosimulation technology-enabled consultancy, announced version 2.0 of its Cardiac Safety Simulator (CSS), which has become a standalone product for the first time. CSS is an alternative to Thorough QT/QTc (TQT) studies.



Evaluating the use of new tumor measurement tools for studies of molecular-targeted cancer therapies.

The need for robust and standardized psychiatric outcomes measures in oncology trials has grown immensely

Faster go/no-go decisions is the primary driver of adaptive-design adoption by sponsors.