
Initiative designed to boost understanding of medical device development in Europe.

Patient focused drug development is playing a larger role in study design and outcome measurement.

New ASCO study evaluating the cancer care system reveals mixed findings.
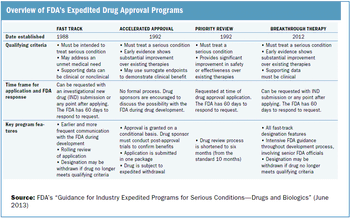
Outlining the requirements and benefits of FDA's four expedited drug approval pathways.

Following four key study-design steps is crucial to the delivery of a successful cross-border cancer study

Trial Design: Breaking Blood Cancer Barriers Adaptive Solutions in Oncology Clinical Technology: Tumor Imaging Ascension Sites: Enrollment Forecast Data Also in this issue: Innovation Initiative Debate in Europe R&D Investment: Public vs. Private The Psychology of Cancer

In yet another demonstration of how far drug pricing concerns are invading the hitherto sacrosanct territory of drug development, a consortium of national advisory bodies on pricing have written to the chief official responsible for European Union drug research.


The European Medicines Agency (EMA), along with the Heads of Medicines Agencies (HMA), have published the ‘EU Medicines Agencies Network Strategy to 2020.

PAREXEL introduced the latest version of its Regulatory Information Management (RIM) platform: LIQUENT InSight® 6.0

Biorasi, a full-service CRO, announced a strategic partnership with Medidata, where it has deployed Medidata's electronic data capture (EDC), management and reporting system, Medidata Rave®, to support Biorasi's Phase I through IV global clinical trial business.




Quintiles and Quest Diagnostics announced a definitive agreement to form a global clinical trials laboratory services business.



Scholarship Program Helps Address Site Sustainability through Education and Training Provides Sites Access to SCRS Member Benefits


Biologic drugs and non-biologic complex drugs have revolutionized the treatment of many difficult-to-treat diseases including cancer, multiple sclerosis, and chronic iron deficiency.

The Society for Clinical Research Sites (SCRS), a global trade organization representing the interests of clinical research sites, announced a two-year partnership with PRA Health Sciences.


Trulicity® (dulaglutide), approved by the EMA in November 2014 for the treatment of Type 2 diabetes in adults, is the first diabetes drug to have a Patient Reported Outcome.

PPD gains real-time and actionable insight into studies with simplified data exchange and monitoring through the cloud

CluePoints, a provider of Centralized Statistical Monitoring (CSM) solutions for clinical trials, and the Alliance for Clinical Research Excellence and Safety (ACRES), a multi-sector non-profit organization dedicated to building a stakeholder-driven integrated global system for clinical research, announced a strategic partnership.




The Alliance for Clinical Trials in Oncology Foundation, a nonprofit organization that supports the mission to reduce the impact of cancer,.
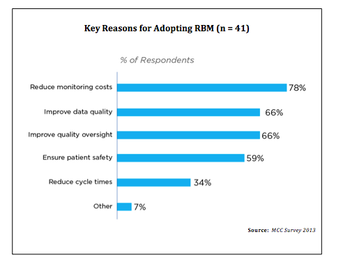
A comprehensive survey by Metrics Champion Consortium