
MyMeds&Me, a provider of SaaS, web-based adverse event and product quality capture solutions for life sciences, announced its collaboration with Oracle Health Sciences to provide the Reportum® solution to Oracle Argus safety customers.

MyMeds&Me, a provider of SaaS, web-based adverse event and product quality capture solutions for life sciences, announced its collaboration with Oracle Health Sciences to provide the Reportum® solution to Oracle Argus safety customers.



Cancer Research UK, a UK-based charitable funder of cancer research, has selected Medidata’s Clinical Cloud® cloud-based technology for EDC and management (Medidata Rave®) for Cancer Research UK’s Center for Drug Development (CDD)-based clinical trials at institutes, universities and hospitals across the UK.

The results from the first Phase 1 trial of an Ebola vaccine based on the current (2014) strain of the virus have been published in The Lancet.


The National Institute of Allergy and Infectious Diseases (NIAID), a component of the National Institutes of Health (NIH), has launched ClinRegs, a public website designed to help clinical researchers navigate country-specific regulatory information as they plan and implement clinical trials.

Vodafone’s Machine-to-Machine (M2M) technology enables PHT Corporation to more efficiently manage worldwide clinical trials through real-time access to patient experience data


goBalto, Inc., the leading provider of cloud-based clinical study startup solutions, today announced its latest version of goBalto Activate.



Biomedical Systems, a premier global provider of centralized diagnostic services, announced its affiliation with the Electronic Patient-Reported Outcome (ePRO) Consortium, a program run by the Critical Path Institute (C-Path).


ACI Clinical announces a new solution for Life Sciences companies facing the growing difficulty surrounding the identification and engagement of independent medical experts.

Series of research projects to evaluate approaches for improving patient understanding of clinical trials


Christa Wirthumer-Hoche, PhD, head of the Austrian Medicines and Medical Devices Agency, has been elected Vice-Chair of the European Medicines Agency (EMA) by the Management Board for a three-year period.

Palatin Technologies is a biotech company focused on developing targeted, receptor-specific, peptide therapeutics for the treatment of diseases with significant, unmet medical needs and commercial potential.

New faces among the Pharma Industry rise the ranks.

Whether we are referring to CSRs or to IPD, the personal information of trial participants needs to be de-identified prior to release. This article will describe the available methods for de-identifying clinical trial data, and the relative strengths and weaknesses of each.




SureClinical Inc., an industry-leader in collaborative cloud health sciences applications, announced today a strategic partnership with Triumph Consultancy Services to broaden its eTMF service delivery portfolio.

To celebrate 20 years since its opening, the European Medicines Agency (EMA) organized a one-day meeting this week. Called “Science, Medicines, Health: Patients at the heart of future innovation”, the invitation-only event in London focused on the EMA’s future role
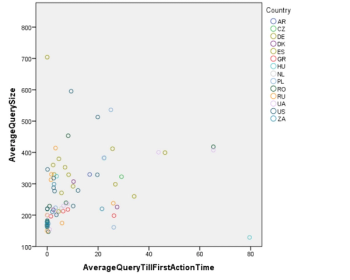
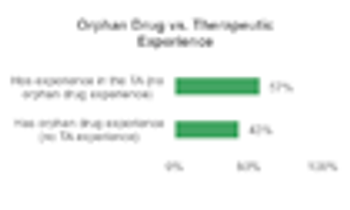
Clinical trial site engagement has been advocated as a critical component relating to a study’s performance and success, however, a minimum amount of data supports this connection.

Annual ASCO Report Shows Widespread Disturbance in Oncology Practice, Amid Growing Patient Demand and Administrative Burden

A new study by the American Society of Clinical Oncology (ASCO) is now available, which chronicles the current realities of the cancer care system and examines trends in the oncology workforce.