
News


PRC Clinical, a CRO specializing in clinical trial management, is joining the Alliance for Regenerative Medicine (ARM).

This whitepaper discusses the importance of observational research and patient registries in evidence generation. The modern healthcare environment is a mosaic of stakeholders, each with remarkably different demands for data addressing product attributes. These often conflicting perspectives require access to a portfolio of interventional and observational research designs sub-serving different objectives … Increasingly central is the inclusion of observational studies, including registries, which provide insights missing from traditional nterventional studies encountered in the course of drug development.

Eisai announced that, in order to further promote transparency in clinical trial data disclosure, it has determined its policy on clinical trial data disclosure and is making clinical trial data publicly available to researchers via an external website.


BioClinica®, Inc., a specialty clinical trials services and technology provider, has partnered with advisory and outsourcing services company Kinapse on BioClinica’s Compass Intelligent Monitoring solution.


inVentiv Health, a global provider of clinical development and comprehensive commercialization services, announced expansion of compliance products and services to assist biopharmaceutical companies conducting non-interventional studies globally.


eCOA provider CRF Health is currently providing two special DIA conference discount offers.

Every trial starts with a budget, and a clinical trial study start-up process that can be fraught with delays. From contract negotiations, to managing a trial for a sponsor, to streamlining study start-up process, this eBook seeks to instruct others on best practices.

It is critical that partners in clinical research develop systems to maximize the potential of big data while protecting the confidentiality of patient information, to further biomedical research, continued the statement, according to a statement from the European Federation of Pharmaceutical Industries and Associations.

Many of us speak about the importance of clinical trial data quality and integrity, yet the lack of data quality standards and definitions introduces subjectivity risk in clinical trials.

Quotient Clinical has announced results from an Enabled-First-in-Human (Enabled-FIH) program conducted for the Janssen WAVE Early Development unit.

Make sure your perspective is heard, and get valuable insights and data for your TMF improvement initiatives



Theorem Clinical Research announced it will open a new, state-of-the-art clinical supplies facility in Frankfurt, Germany, in June.
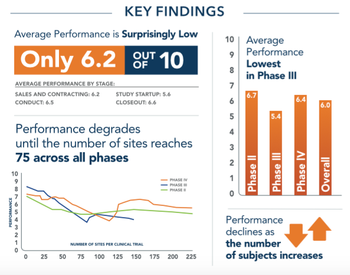
Graphics and data regarding the current state of the Clinical Trials industry.

Type 2 diabetes mellitus is one of the Western world’s most common chronic conditions, with global prevalence increasing rapidly.

New faces among the Pharma Industry rise the ranks.

ClinicalTrials.gov is a federally mandated database with a large and growing, number of required data fields. Organizations are required to report any study being conducted under FDA auspices. One of the mandated reporting fields is the date that the FDA receives notification of a clinical trial’s initiation, with less than 3% of the studies in the database missing that information.

Radiant Sage, a provider of on-demand clinical trial imaging infrastructure solutions, announced its Event Adjudication Committee (EAC) solution for its RadClinica Clinical Trial Management System (CTMS).


CRO Analytics, a provider of validated clinical trial performance data, announced a partnership with the Association of Clinical Research Professionals focused on measuring investigator site personnel views of clinical trial quality




With the introduction of mobile health technologies in the field of healthcare, Sponsors and CROs are looking into mHealth to design patient centric clinical trials in order to reduce study visit costs and trial participation burden on patients.
