
The report presents a construct of four levels of open innovation.

The report presents a construct of four levels of open innovation.

PPD and HealthCore, the clinical outcomes research subsidiary of Anthem, have established a collaboration that will enable both companies to further expand their services.


PRA Health Sciences announced its new Predictivv™ platform, a fully integrated solution for designing, planning and optimizing the management of global clinical studies.

CRF Health, a global provider of electronic Clinical Outcome Assessment (eCOA) solutions for the life sciences industry, announced the introduction of TrialMax ConMed™.



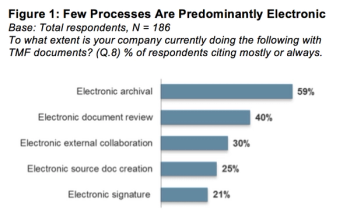
Industry-wide survey shows eTMF applications improve inspection readiness and seen as key to shortening development time.


BBK Worldwide has appointed Toshio Mori to Managing Director of Operations, Japan. Mori will lead business development and help drive sales and marketing as well as establishing and growing the company’s new office based in Tokyo, Japan.



BBK Worldwide, a clinical trial marketing firm, announced My Research Mate, a live streaming video app platform developed to educate consumers about treatment options and available clinical research studies.



Parallel 6, mobile engagement and management solutions provider, and Sentrian™, a Remote Patient Intelligence company announced its technology partnership.

Medidata, a global provider of cloud-based solutions for clinical research in life sciences, announced that PROMETRIKA, LLC, a full-service clinical research organization.

Comprehend Systems, a clinical software solutions provider, announced the launch of Continuous Quality for ClinOps.


ArisGlobal, a provider of cloud solutions to life sciences companies, announced it has acquired Medsight Solutions, which includes its integrated risk management solution, Medsight Sapphire.



ERT, a global provider of patient safety and efficacy endpoint data collection, cloud analytics and workflow solutions, announced that MasterScope® 2, its comprehensive diagnostic platform for spirometry, electrocardiogram (ECG) and home monitoring, has been successfully implemented with 350 investigative sites.

ArisGlobal, a provider of cloud solutions to life sciences companies, announced agReporter a solution for users to correspond with drug manufacturers and get the latest medical advice.


Version 3.0 of the TMF Reference Model incorporates feedback from its extensive industry use to enhance content clarity.


New faces among the Pharma Industry rise the ranks.

New portal solution allows investigators to access, sign, and upload eTMF documents on site.