
This new version release optimizes patient recruitment with study-specific prescreening workflows and a delivery platform that allows for self-administered cognitive assessments on any modality including tablet-based assessments

This new version release optimizes patient recruitment with study-specific prescreening workflows and a delivery platform that allows for self-administered cognitive assessments on any modality including tablet-based assessments


Strong Focus on New Research Collaborations; Up to 130 New Positions to Be Created.

The Pediatric Committee (PDCO) of the European Medicines Agency (EMA) has revised the current list of class waivers for medicines that are not required to submit a pediatric investigation plan.


LabCorp® announced that Covance Drug Development has relocated its clinical research unit in Dallas, TX, to a new, fit-for-purpose clinical research facility.


This following list comprises all of our most popular Tweets from 2015. Find out what made the cut and read more about trending topics regarding Clinical Trials.Follow @Clin_Trials for more information!

Collaboration Enables Use of Actionable, Patient-Generated Data in Clinical Trials from Extensive Range of mHealth Sensors, Wearables and Apps


Collaboration Enables Use of Actionable, Patient-Generated Data in Clinical Trials from Extensive Range of mHealth Sensors, Wearables and Apps

Quorum Review IRB is introducing a comprehensive program that supports the needs of Phase I Healthy patients.

With the Perceptive MyTrials Analytics solution, clinical trial sponsors can now use a mobile-enabled, single entry-point to access predictive data analytics.


To mark the 50th anniversary of the adoption of the first European Union (EU) pharmaceutical legislation, the European Commission is organizing a one-day conference that will take place in Brussels September 28.




Wingspan Technology now offers an eTMF Readiness Assessment program to help sponsors and CROs assess their level of readiness for implementing eTMF.


According to the release, PPD noted that wearable technology will have the potential to benefit Novartis and its other clients because of the integration with the technologies patients use on a daily basis.

The aforementioned article emphasized that a reduction in data quality ultimately leads to enrolling more clinical trial subjects in order to maintain equivalent statistical power.

The program is designed to facilitate dialogue between industry stakeholders and clinical research sites.


In light of two recent announcements, and this excellent article from Bloomberg it appears the role of social media in post-market adverse event reporting is being taken seriously by the FDA.
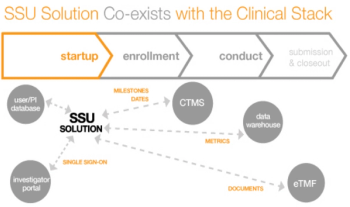
The need for more efficient clinical trials is driving greater use of cloud-based solutions, especially with the rise in globalization


Designed to ensure patients and patients in clinical trials safely receive their proper medications, the regulation serves as a verification measure to enforce the European Union’s Falsified Medicine Directive.
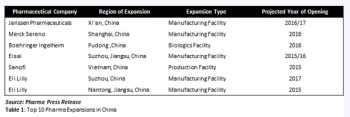
In this analysis, distinctions between who hires who in non-Chinese and China-based companies emerges.

The new Clinical Conductor contains a myriad of enhancements that foster more precise reporting and analysis.