
Kayentis, a French software provider specialized in solutions for clinical trials, has chosen SFR Business Team for its M2M roaming in close to 250 countries.

Kayentis, a French software provider specialized in solutions for clinical trials, has chosen SFR Business Team for its M2M roaming in close to 250 countries.

The U.K. National Health Service (NHS) has granted approval for what is thought to be the first use of electronic informed consent in England.

As the push to contain clinical trial costs and make informed business decisions continues, more pharma and CROs are looking at internal and external benchmarking and metrics to evaluate their performance-both successes and challenges. This e-Book includes articles that show how analytics uncover problem areas in clinical operations, as well as metrics and databases that are used to pinpoint internal or external performance issues.

An article recently appeared in Applied Clinical Trials, which indicated that oncology trials are taking longer to complete, and the article suggested that the duration of oncology clinical trials in certain phases increased by one year to one and a half years.1 Moreover, the article delineates that ameliorations in trial duration were most likely a result of increasing protocol complexi

Across the pharmaceutical industry, advances in digital technology play a key role in driving the efficiency of the drug development process, particularly for clinical trials.


The Top 5 Risk-Based Monitoring Articles

INTERLAB and synlab have been successfully cooperating since 2004.


Will emerging drug cost assessment initiatives provide useful, valid information on total drug value?

This experimental technique is being studied to see whether it could treat certain health problems.


Admedus adopts the Medidata Clinical Cloud® to bring greater speed and efficiency to it’s genital herpes therapeutic vaccine study.

Implementation establishes foundation for sustainable growth in early phase clinical research


Clinical trials are time sensitive, costly endeavors with high-risk and high-expectations at every juncture.


CRF Health, a provider of electronic Clinical Outcome Assessment (eCOA) solutions, has been selected as a partner of choice by two top tier pharmaceutical companies.


KMR Group reports that the duration of clinical trials continues to hold steady or increase despite ongoing efforts by biopharmaceutical companies to reduce cycle times.


U.S. Representative Billy Long has introduced legislation to update regulations guiding how drug manufacturers communicate product information.
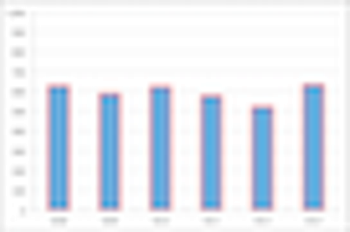
Randomized Clinical Trials (RCTs) have constituted the foundation for new drug approvals for over fifty years.

This month marks a landmark in the quest for personalized medicine and the growing role of big data in health sciences – from clinical development through tracking patient outcomes long after a therapy has reached the market.

One of the worrisome aspects of the U.S. Open Payments transparency program is that it may discourage doctors from serving as investigators in clinical studies. The two-year-old program requires public disclosure of payments by drug and medical device manufacturers to health care professionals (HCPs) and teaching hospitals for conducting clinical trials, as well as for marketing and consulting activities. But an anomaly in the program credits to the principal investigator (PI) heading up a research site the full payment for managing and carrying out the study at that site.




