
News


Tufts CSDD finds drug and diagnostics companies are investing in the development of companion diagnostics that can show that their use in conjunction with personalized therapeutics will lead to positive health outcomes.

An analysis by Life Science Compliance Update of the U.S. government's Open Payments database shows that industry spending on U.S-based clinical research has dropped 32% in the first year-over-year comparison since Open Payments data started to be collected.




Kowa Company Ltd, a Japanese pharmaceutical company, has migrated to Oracle Argus Cloud Service Global and Oracle Argus Cloud Japan.

New faces among the Pharma Industry rise the ranks.

How can you navigate the regulatory triangle safely?


The Jumpstarting Brain Tumor Drug Development Coalition (JBTDDC) announced a new consensus protocol for magnetic resonance imaging (MRI) in brain tumor clinical trials to help assess if a new treatment is effective.


CRO is the first to participate in IACOR’s accreditation pilot.


Cloud-based solution allows investigators to simplify the development of clinical research protocols.



The New England Journal of Medicine recently published results of a multi-center Phase II clinical trial, managed by CTMG.

BioClinica®, a clinical trials services and technology provider, announced the launch of the BioClinica eHealth Cloud™.

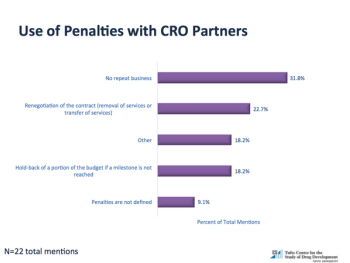
Demand for outsourced services to provide clinical development capacity and expertise has grown substantially over the last 10 years.
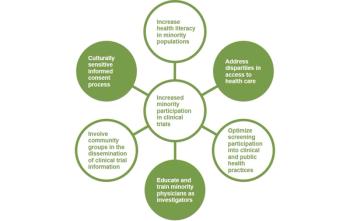
Promising new approaches to optimize data and outcomes.
Few topics occasion the lamentations of clinical trial professionals more than the topic of patient inclusion/exclusion criteria in clinical protocols.


The identification of concomitant medication poses specific challenges in oncology data reporting.



Chiltern, a global CRO headquartered in the UK, announced it has signed a purchase agreement to acquire Theorem Clinical Research, based in Wilmington, NC. The combined company will operate under the name Chiltern and will gain global reach - most notably in China and Japan - as well as new service offerings for medical devices and diagnostics, clinical analytics and clinical supplies. The transaction is expected to close within the next several weeks.

