
An analysis of 10.2 million adverse report records filed with the FDA along with survey of 123 health professionals by Tufts CSDD, found voluntary adverse drug event (ADE) reporting in the United States is incomplete, inaccurate, and inefficient.

An analysis of 10.2 million adverse report records filed with the FDA along with survey of 123 health professionals by Tufts CSDD, found voluntary adverse drug event (ADE) reporting in the United States is incomplete, inaccurate, and inefficient.




New faces among the Pharma Industry rise the ranks.


ICON announced that it is applying IBM's Watson Clinical Trial Matching to its breast, lung, colon and rectal cancer trials.


Certara® and Paidion Research, announced a new partnership to address the challenges inherent in pediatric drug development.


ACRP believes the arbitrary two-years experience generally required throughout the industry is contributing to a shortage of CRAs and negatively impacting efficiencies and quality in clinical research.

The analysis compared various manifestations between genotypes and to determine if correlations exist between TSC symptoms and the TSC gene mutation.



Medidata, a global provider of cloud-based solutions for clinical research in life sciences, announced that its Clinical Cloud platform has been adopted by Walvax Biotechnology.
We all need help sometimes. For pharmaceutical companies, this often comes in the form of a reliable and competent contract research organization.


CRO embarks on the company’s largest-ever federal project.

Almac announced the expansion of its shipping service for clinical trials to EU countries.


The US ophthalmology-focused CRO will use the solution to provide real-time key operational parameters.



The world of RBM is continually changing as biopharmaceutical enterprises are dabbling into different approaches and methods. There are several outsourced models that exist, when approaching RBM, such as integrating cloud-based solutions to provide centralized monitoring teams with analytics, or fully outsourcing RBM functions and technologies to CROs. However, some companies, such as Novartis, appear to be in-sourcing their RBM technologies and functions.

BioClinica’s eHealth App xChange provides access to an ecosystem of integrated mobile and cloud applications.

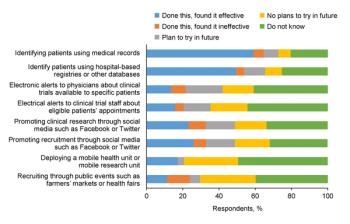
A staggering number of clinical trials fail to meet recruitment goals, which leads to delays, early trial termination, or inability to draw conclusions at trial completion due to loss of statistical power.

PPD and CISYS LifeSciences announced PPD’s implementation of Sequence WebEAS, a new Web-based event adjudication system, through a collaboration between the two companies.

