
News



The Cantrixil Phase I study will be weighted towards ovarian cancer patients with the selection of a gynecological oncology site.




Ethical GmbH’s Ethical eAdjudication® Platform will be included in ACRES’ shared global platform of integrated technologies.

As cancer immunotherapies continue to emerge, the need for rigorous evaluation to assess the safety and efficacy of these products in clinical trials is critical. Doing so in the early phase setting requires a foundational focus in such areas as NME selection, protocol development, patient population, and investigator and site selection.

The industry is experiencing a paradigm shift from pharmacovigilance rooted in case processing and compliance reporting to a safety program built around benefit-risk management.

TRI will be providing the OPRA platform for all of Advanced’s studies whose sponsors have requested a risk-based approach to monitoring.


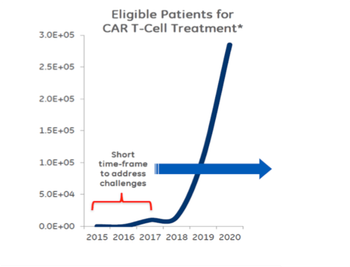
Without question, advanced cellular and gene therapies require well-defined cold-chain management solutions that reduce risk and include all elements of packaging, data collection and logistics expertise to ensure high-quality, effective treatments reach the point of care, and ultimately, the patient.






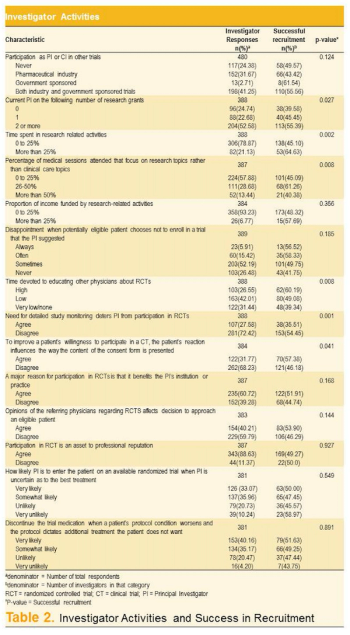
Data on the relationship between investigator characteristics and cardiovascular clinical trial patient recruitment are extremely limited in scientific literature. An analysis of ALLHAT, the largest antihypertensive clinical study ever conducted, identifies key investigator and site characteristics that can strengthen subject enrollment.

Accruing patients depends greatly on engaged, enthusiastic PIs, as they are the gatekeepers in helping patients decide to participate in clinical trials. Building staff awareness and enthusiasm are also essential for retaining participants.




Parexel announces PAREXEL® Access, a service that is directed toward clients’ market access and ongoing commercial success.


Japan is increasingly a region where opportunities for global registration studies abound, supported by government policies as well as the medical community and its local population. However, incorporating Japan as an integral part of your global trial execution strategy does not come without its unique challenges.

