
The solution, DLTA, is built on a project-management platform overlaid with knowledge of drug development and FDA requirements.

The solution, DLTA, is built on a project-management platform overlaid with knowledge of drug development and FDA requirements.
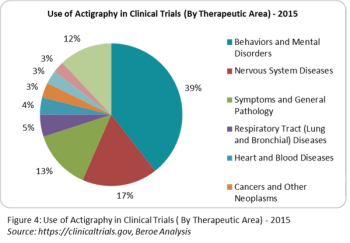
With all the buzz around wearables, actigraphs-which monitor sleep and daytime activity levels-have been around for decades.
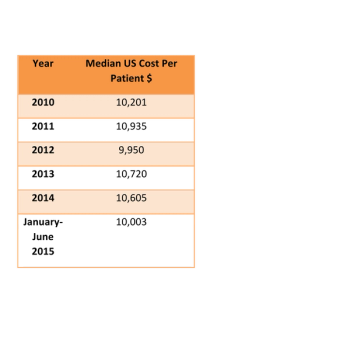
A substantial increase in procedure complexity should be reflected in increasing per patient costs of Phase III clinical trials,which is not true from this data.

With the initial promise of immunotherapy use in oncology, we need to continue supporting cross-industry collaborations among leaders from the biopharma community, academia, regulators, and private sector investors.

An investigation published by the BMJ this week has raised new concerns about the validity of a trial that was used to gain approval for Xarelto from the U.S. and European regulators.

The study gathered responses from more than 12,000 people around the world--including 3,000 study volunteers--about their views and experiences with clinical research.

The facility features space for ambient, refrigerated, cold and ultra-low temperature storage as well as liquid nitrogen vapor phase storage with storage capacity for more than 8 million samples.




ONO will be conducting a series of clinical trials in Japan to assess the safety and efficacy of using OPDIVO for a wide range of additional indications, including head and neck cancer.

GuideStar will collaborate with ACRES on its Site Accreditation Standards Initiative as a Strategic Ally.


Radiant Sage has partnered with Oslo, Norway-based imaging CRO Clearpoint Image Review Center to provide expanded imaging operations.
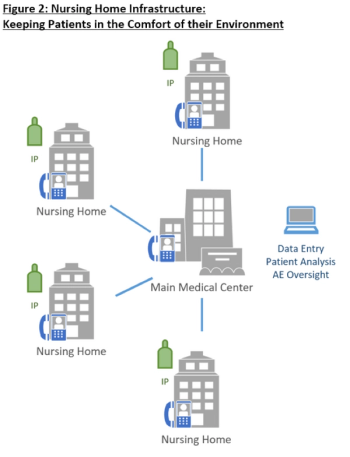
This case study describes how innovative structural design at a study site is leveraging innovative telehealth technologies and nursing home infrastructures to recruit and retain Alzheimer’s patients.

Because identifying and communicating with patients who are willing to participate in trials can be a significant challenge, Synexus says it is keen to adopt a streamlined process that offers consistency across multiple channels.


Slightly more than a thousand healthcare research projects were funded by the European Union between 2007 and 2013, at a cost of $6 billion, revealed Carlos Moedas, European Commissioner for Research, Science and Innovation, at the end of January.

New approach focuses on assessing the effectiveness of four key areas related to risk-minimization and pharmacovigilance activities in Europe.
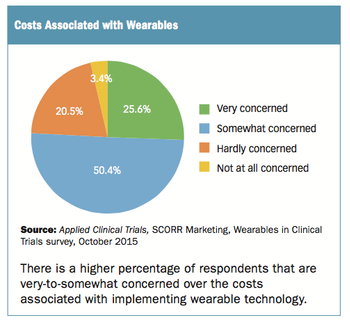
Survey spotlights the concern levels associated with the adoption of clinical trials using wearable technology.

Study shows sharp reduction in market exclusivity periods for first-in-class treatment entrants.

Search-engine data offers window to public consciousness around clinical trial research, participation.

This three-step process can help managers accurately assess how patient-centric their trials really are.

Despite wealth of data collected, many inefficiencies exist in the site-sponsor transfer of insights from clinical trials.
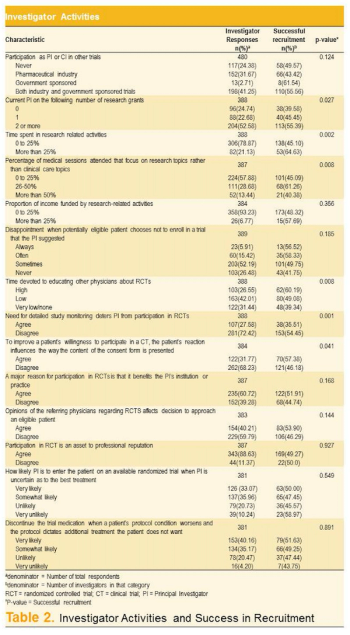
Analysis of the largest antihypertensive trial ever offers useful blueprint for sites in boosting subject enrollment.

Survey takes deep dive into enrollment hurdles, aims to inform future recommendations for improvement.
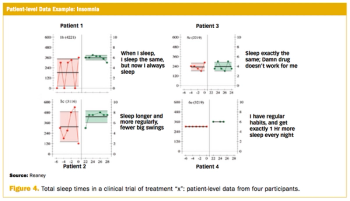
Measuring clinically relevant response requires routine and regular collection of outcomes data.

Click the title above to open the Applied Clinical Trials February/March 2016 issue in an interactive PDF format.

French safety authority confirms what had been speculated--Phase I volunteers received the fifth of the highest dose escalation of the investigational drug at the same time, which goes against EMA recommendations.
