
News


The success of a clinical trial depends on the sponsor’s ability to effectively execute its programs. Both quality of research and aptitude to complete the study on time determine the likelihood of advancement of a product to the next stage of development.

Clinerion and the The International Clinical Trial Center Network (ICN), a collection of academic and government-based research institutions, announced the adoption of Clinerion’s Patient Recruitment Service for ICN’s members. This system brings medical informatics solutions for protocol design, site selection and patient recruitment.










Mike Graziano, VP of Toxicology at Bristol-Myers Squibb, sits down with Moe Alsumidaie to discuss BioCelerate’s toxicology data sharing initiative.



Catch up on who has new jobs and recognitions that companies have received.

The Geriatric Medicines Working Party of the EFGCP is organizing a workshop on “Technology, Ethics and Older People in Clinical Research.” The goal is to explore what can be done to involve older people in clinical trials.





All the IT systems of the European Medicines Agency (EMA) will be temporarily unavailable from 6 am U.K. time on Saturday, April 16 to 6 am Sunday the 17th (U.K. time) due to essential maintenance, according to an EMA announcement released yesterday.


OmniComm has announced the signing of an agreement with Medistat Ltd.

SCRS announced that Medpace will participate as a Global Impact Partner dedicated to promoting communication and collabaration.


Bio-Optronics has announced the launch of their new Clinical Conductor CTMS product line. This update enables users to modify the software homepage to suit their needs and adapt with their workflows.

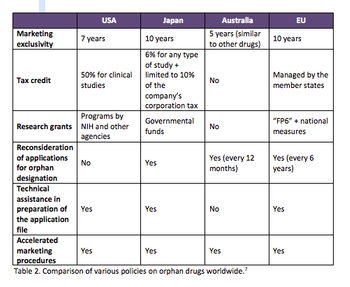
Orphan medicines have become a formidable R&D segment with robust growth that is expected to continue. Expansion is needed to cope with the issues of development and patient recruitment. An understanding of regulations, therefore, is critical.
