
News


Veeva Systems introduced Veeva Vault CTMS to its suite of clinical applications.
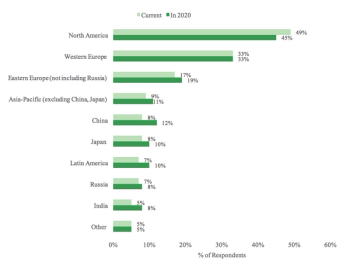
Report shines light on the current state of the Phase II/III clinical trial market and its anticipated direction into 2020.


Medidata and the Southampton Clinical Trials Unit (SCTU) at the University of Southampton have announced a partnership.


Patient recruitment company BBK Worldwide has partnered with Apptomics to develop a new suite of mobile applications for patients, caregivers, physicians and payers.



Commonwealth Informatics announced that it has been contracted by the FDA to provide consulting services and technical assistance.

Oracle announced that Pfizer has selected Oracles Cloud Services across its clinical trial portfolio.


Green Circle Health and Integrated Clinical Solutions announced a partnership to provide a passageway through which patient-reported data can be communicated with a trial sponsor or agent.


IMS Health has acquired Privacy Analytics to extend its real-world evidence capabilities.


Catch up on the latest business and people news in the industry today.


Bioclinica announced the debut of its two new divisions, creating a combined offering focused on patient engagement.



ActiGraph announces the upcoming launch of the CentrePoint Data Hub.



WIRB-Copernicus Group (WCG) announces an agreement with karmadata to acquire applications that facilitate trial data management.


ERT introduces its Centralized DLCO Services for assessing diffusing capacity of the lungs for carbon monoxide data in respiratory trials.

LSK Global PS announces that it will expand its use of Medidata’s Clinical Trial Management Solution.

An updated release to the ICH E14 Guidance could indicate that data be used to replace a TQT trial for regulatory submission and review. The result of such a change would be a reduction in development time and costs for biopharma companies.