
News


CROs H2O Clinical and Pharma Start have announced a merger to continue providing biostatistics, data management, clinical operations and medical writing to the pharmaceutical, medical device and biotechnology industries.

A statement issued by the European Forum for GCP (EFGCP) states that provided further updates are made, the ICH guideline for Good Clinical Practice (E6) can stay relevant for another 20 years.


TrialScope announces the addition of four new customers who have all contracted for PharmaCM, TrialScope’s SaaS-based clinical trial disclosure platform.

With the ever-present gap in ability to recruit patients into clinical trials, emerging solutions take different tactics to find the right patients and enroll them quickly.
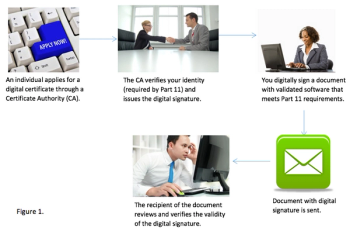
With the healthcare industry’s transition from paper to digital, verification is changing beyond that of a signature in ink. Electronic signatures are a new medium in clinical trials that the industry is now turning to in this transition to digital.

The new facility replaces the company’s current Moscow depot and increases its size and capabilities--including local sourcing of comparator drugs and secondary packaging and labeling.


While the clinical trials industry is currently examining mHealth technology pilots, the healthcare industry has developed them to the point of full deployment. Mon Weschler of Montefiore elaborates on his experiences successfully piloting and deploying these technologies.

The selection of study endpoints is one of many risks that a clinical research study can face. An independent review can help avert this by identifying potential issues within clinical trial data early in the study and allowing the study team to monitor, intervene and reduce study risks.


PPD announces the acquisition of Evidera, an evidence-based solutions provider.

TransCelerate has launched 11 informational programs through its Site Qualification and Training (SQT) initiative to improve understanding of clinical operations for new site personnel. Katarina Hugeneck of Eli Lilly and Theresa Stewart of Allergan discuss the SQT initiate with Moe Alsumidaie.


OmniComm Systems announces the selection of its Trial One platform by a pharmaceutical company to automate its Phase I and BA/BA clinic.
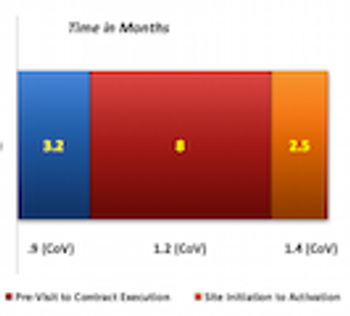
Study finds that despite the use of new approaches to streamline and accelerate study start and improve site selection and activation, the impact on start-up cycle times has been limited. The reasons why are explored.


The Avoca Group’s Quality Consortium’s fifth annual Summit took place in May and June of this year bringing together over 250 attendees from 50 companies. This years event brought out unifying themes around innovation and patient centricity.

Quintiles and DaVita Clinical Research have allied to provide capabilities and solutions for renal disease clinical trials.

The Society for Clinical Research Sites (SCRS) has expanded its relationship with inVentiv Health.



The EMA has published a final report about its pilot project on adaptive pathways. The results of the project showed that adaptive pathways can bring together regulators, HTA bodies, healthcare professionals and patients to agree on plans to generate data on a medicine in areas of unmet medical need.

Medidata announced that its cloud technology platform has been adopted by Israeli biotech, Pluristem Therapeutics.

In the shifting landscape of product development, the pharma and biotech arenas increasingly emphasize the need to use less internal R&D resources, increase efficiency, and reduce costs. One product development strategy is the utilization of lean outsourcing models for clinical studies.

Despite the increasing number of clinical trials in the United States, still participation remains at an all-time low. You can make a difference with participation in INC Research and CISCRP’s “Inspiring Hope’ Ideathon by submitting ideas to increase clinical research awareness.


OmniComm Systems announced that it has signed an agreement with one of the top five medical research universities in the United States.